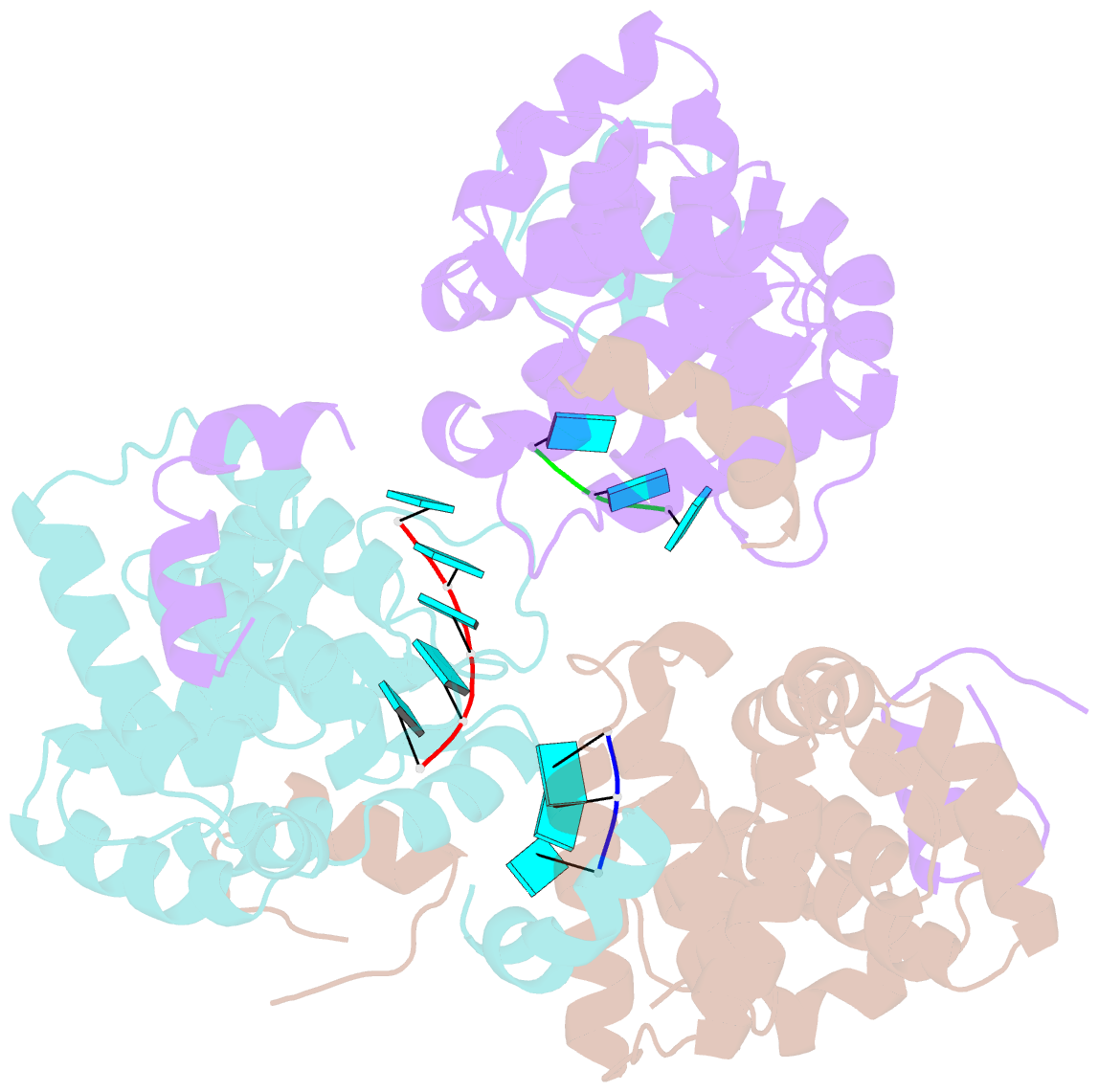
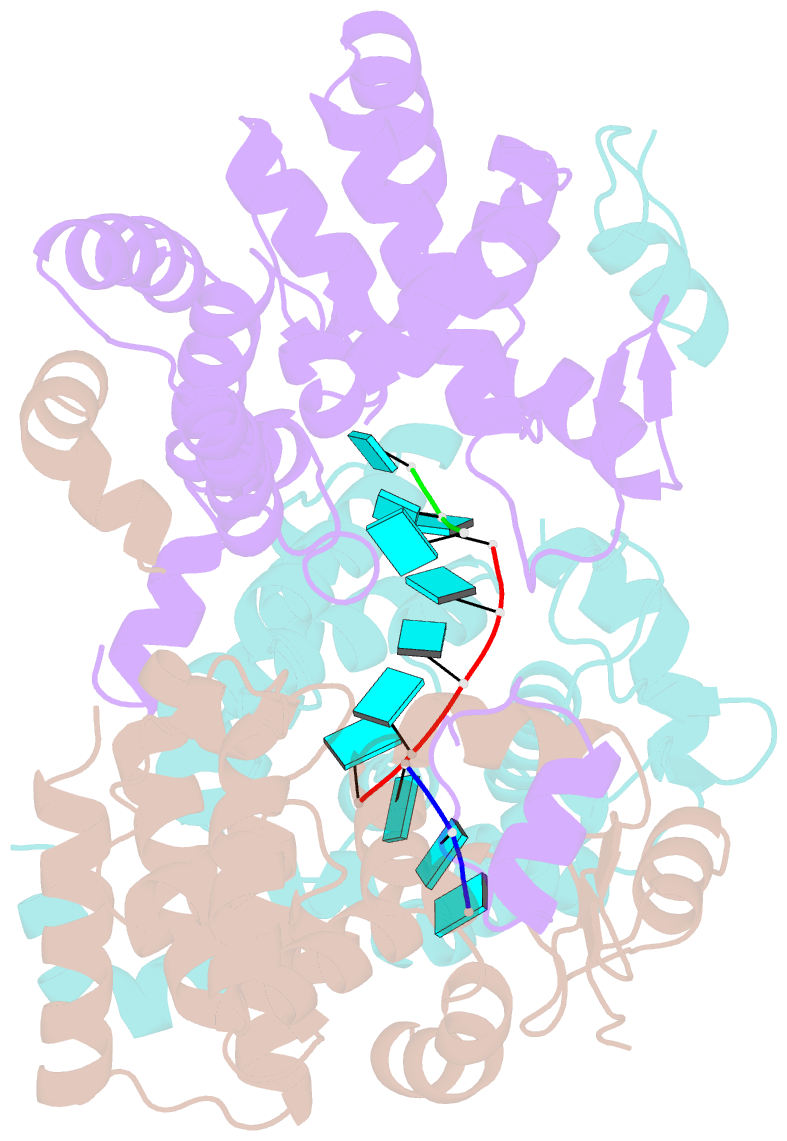
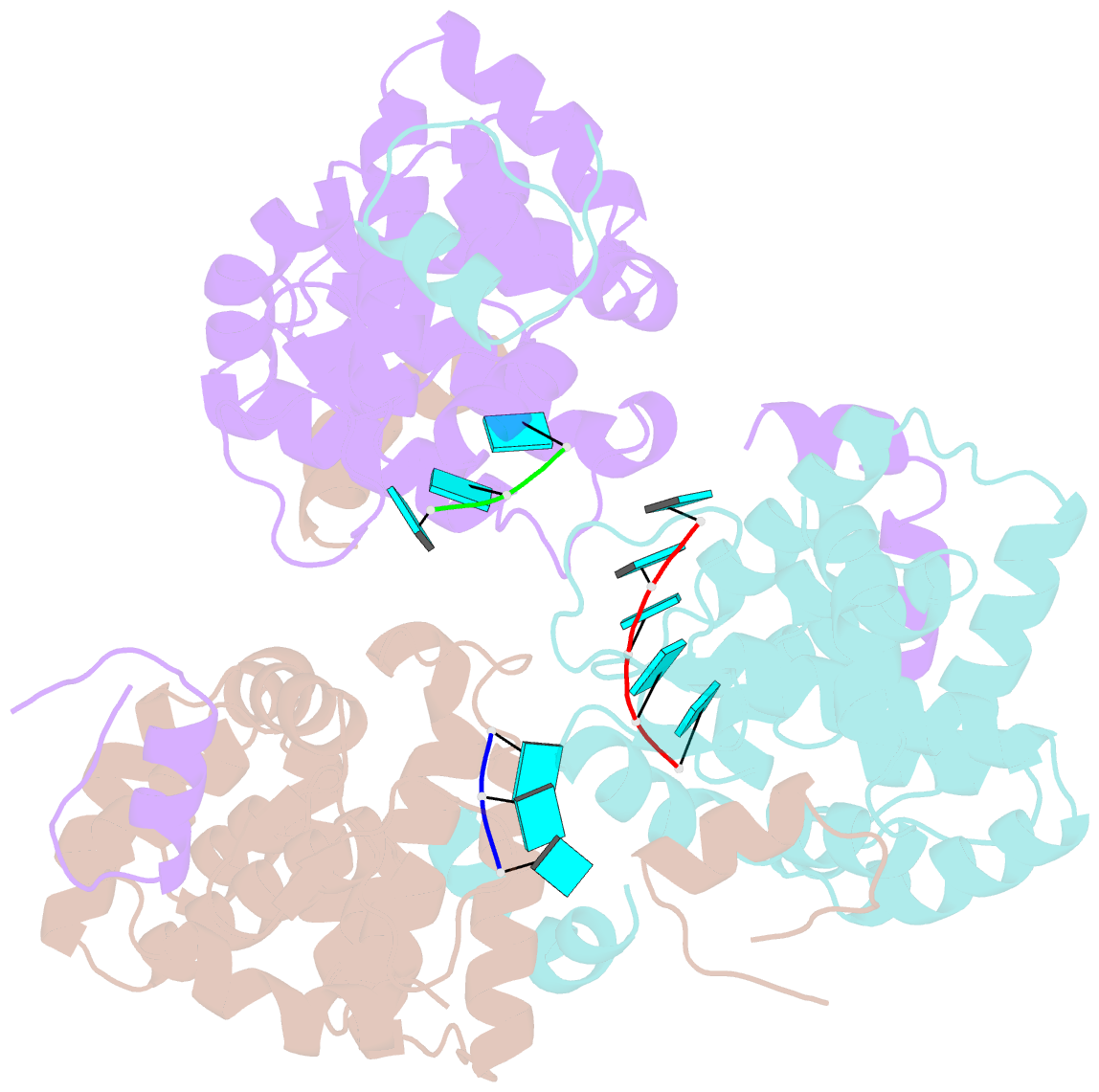
Summary information and primary citation
- PDB-id
- 5ip2; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- viral protein-RNA
- Method
- X-ray (3.3 Å)
- Summary
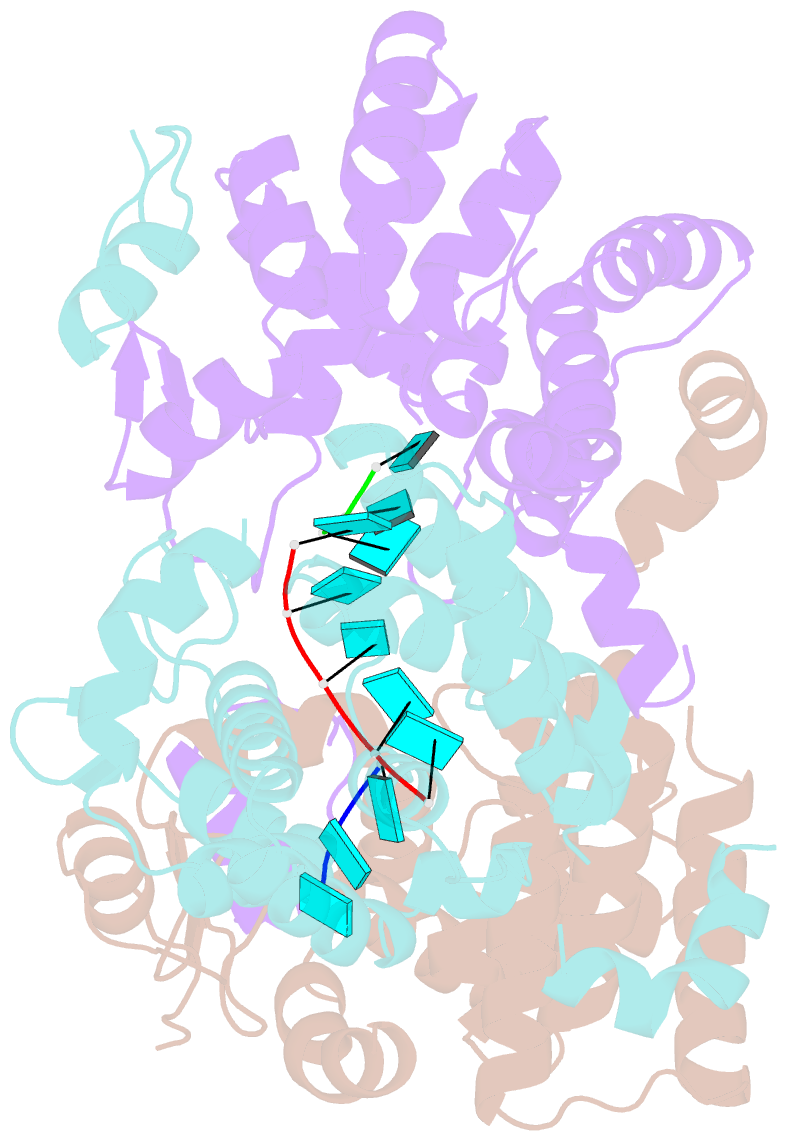
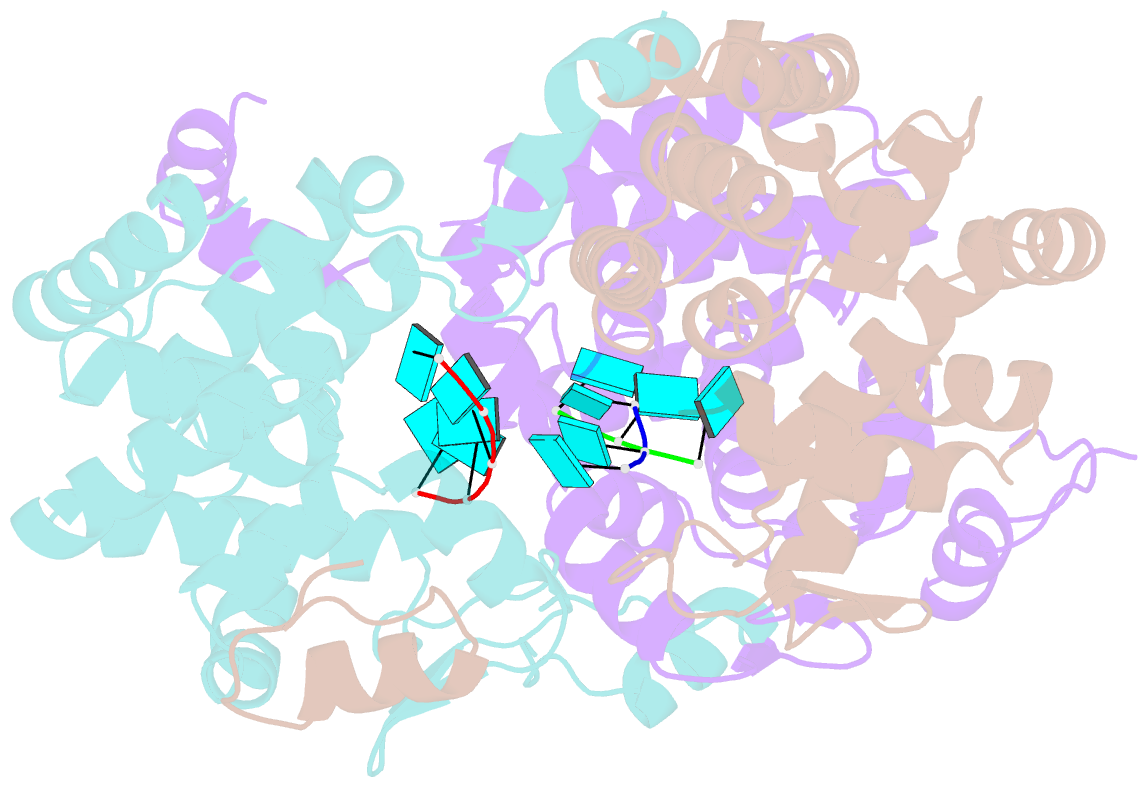
- Tomato spotted wilt tospovirus nucleocapsid protein-ssrna complex
- Reference
- Komoda K, Narita M, Yamashita K, Tanaka I, Yao M (2017): "Asymmetric Trimeric Ring Structure of the Nucleocapsid Protein of Tospovirus." J. Virol., 91. doi: 10.1128/JVI.01002-17.
- Abstract
- Tomato spotted wilt virus (TSWV), belonging to the genus Tospovirus of the family Bunyaviridae, causes significant economic damage to several vegetables and ornamental plants worldwide. Similar to those of all other negative-strand RNA viruses, the nucleocapsid (N) protein plays very important roles in its viral life cycle. N proteins protect genomic RNAs by encapsidation and form a viral ribonucleoprotein complex (vRNP) with some RNA-dependent RNA polymerases. Here we show the crystal structure of the N protein from TSWV. Protomers of TSWV N proteins consist of three parts: the N arm, C arm, and core domain. Unlike N proteins of other negative-strand RNA viruses, the TSWV N protein forms an asymmetric trimeric ring. To form the trimeric ring, the N and C arms of the N protein interact with the core domains of two adjacent N proteins. By solving the crystal structures of the TSWV N protein with nucleic acids, we showed that an inner cleft of the asymmetric trimeric ring is an RNA-binding site. These characteristics are similar to those of N proteins of other viruses of the family Bunyaviridae Based on these observations, we discuss possibilities of a TSWV encapsidation model.