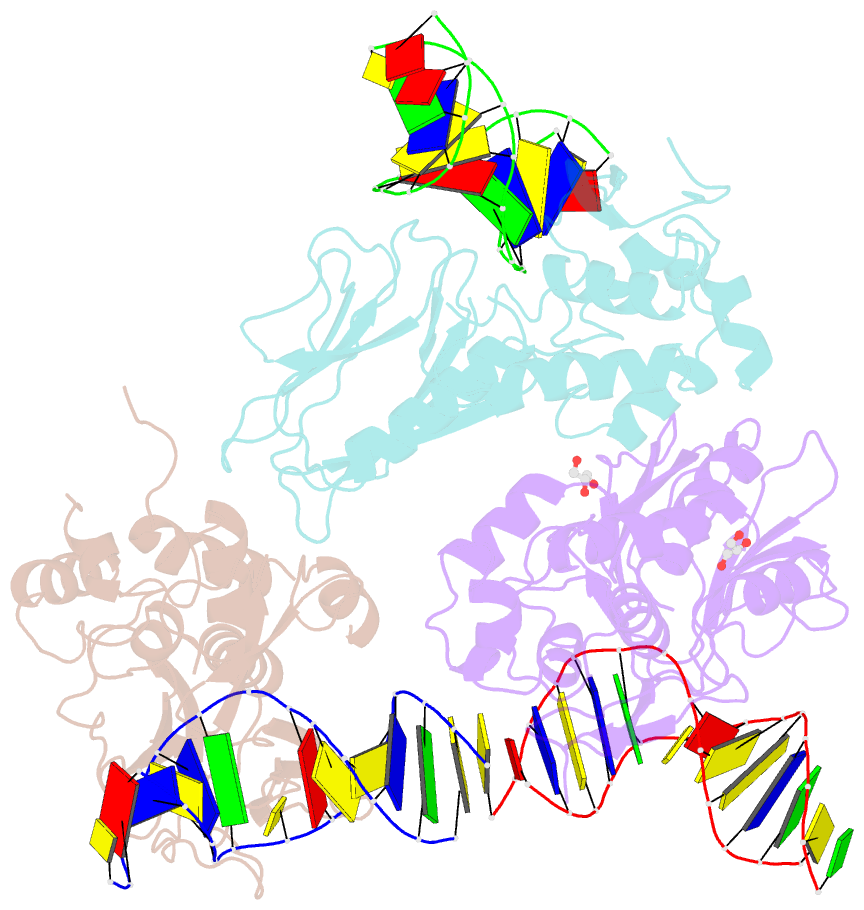
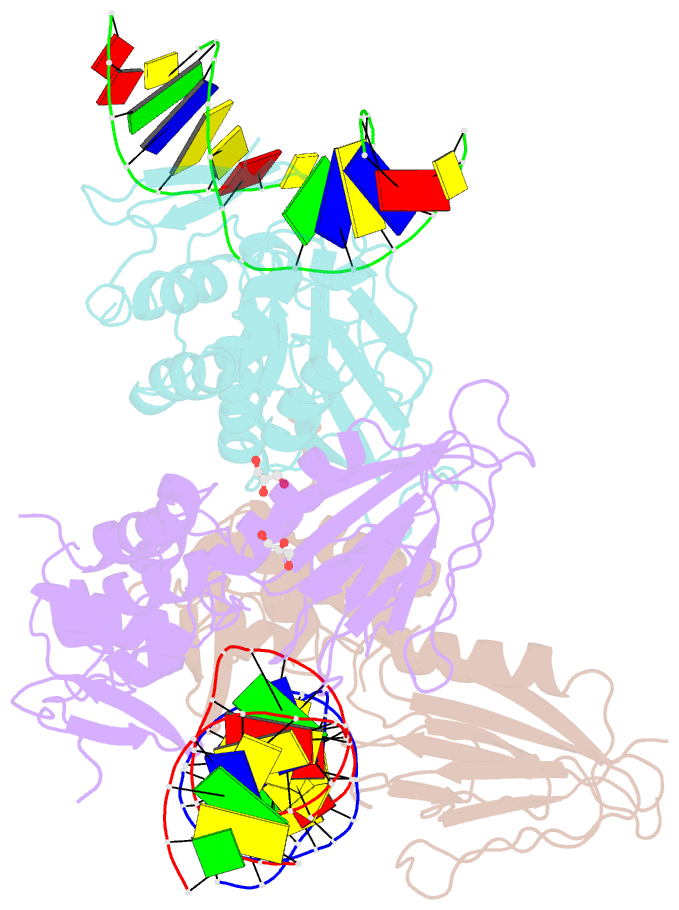
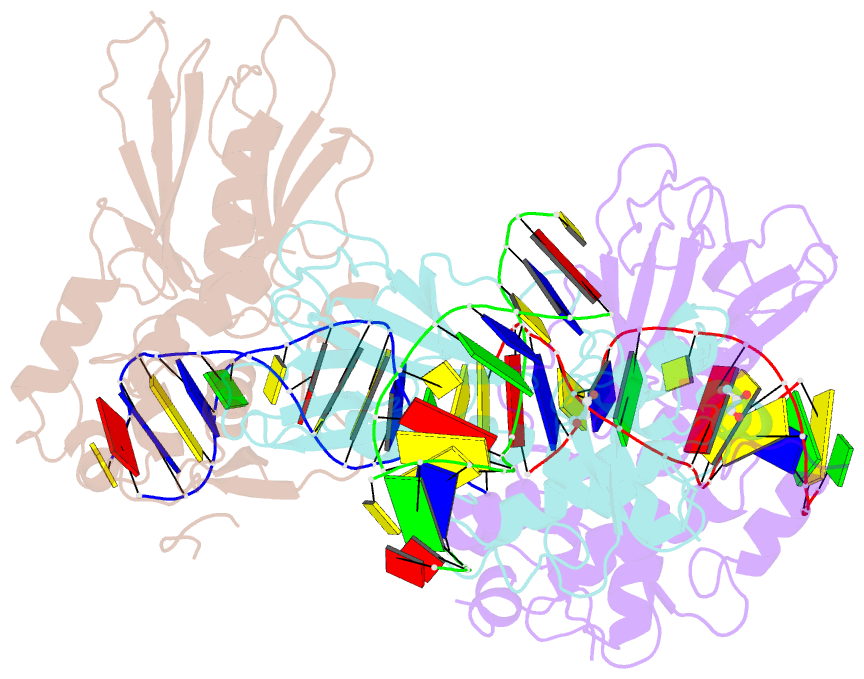
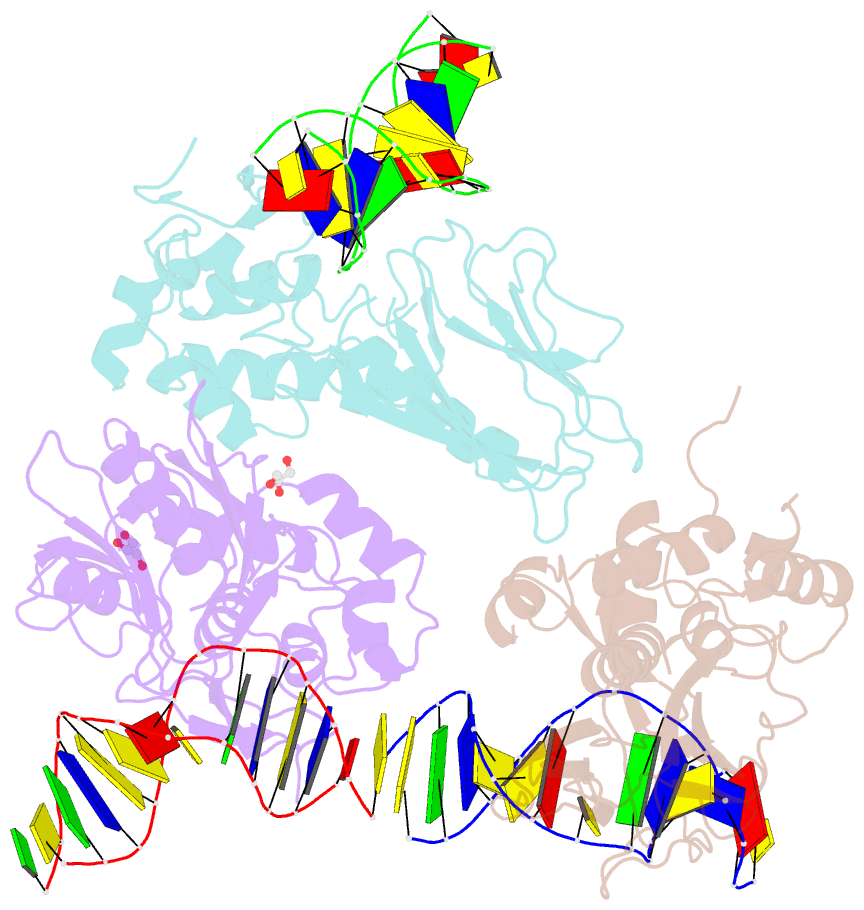
Summary information and primary citation
- PDB-id
- 5itt; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-DNA
- Method
- X-ray (2.53 Å)
- Summary
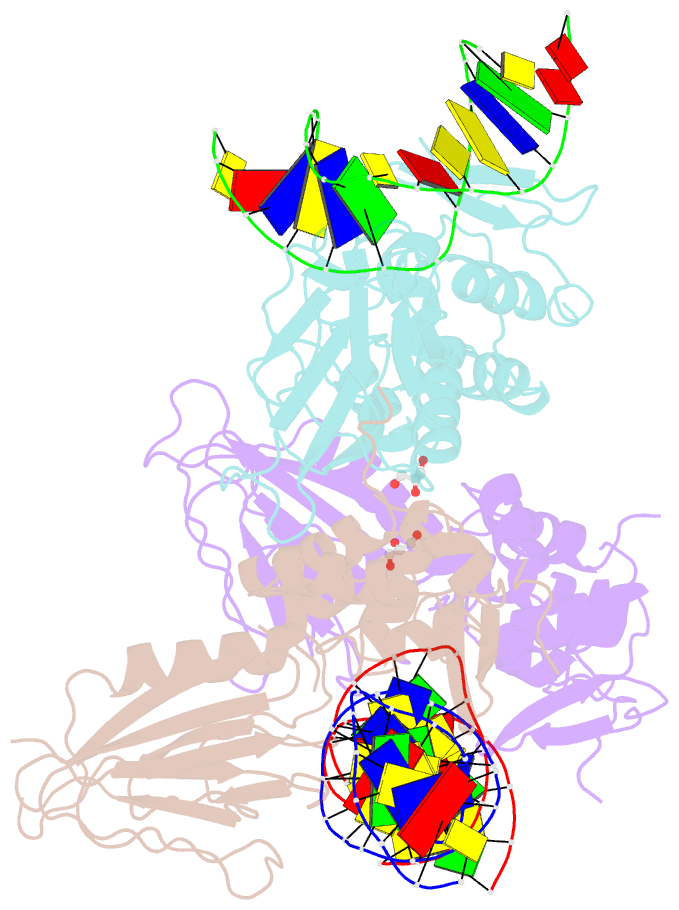
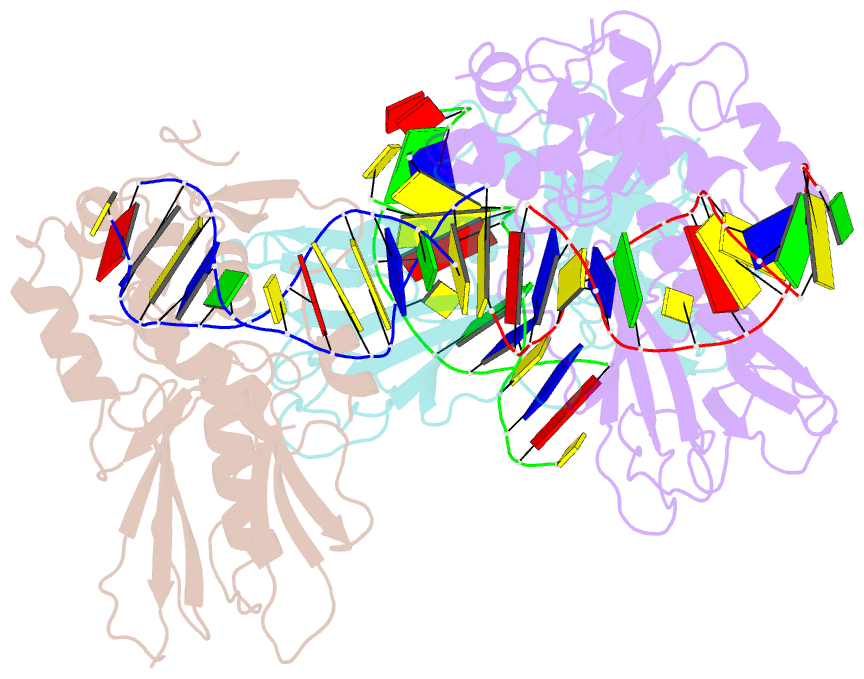
- Crystal structure of human neil1 bound to duplex DNA containing thf
- Reference
- Zhu C, Lu L, Zhang J, Yue Z, Song J, Zong S, Liu M, Stovicek O, Gao YQ, Yi C (2016): "Tautomerization-dependent recognition and excision of oxidation damage in base-excision DNA repair." Proc.Natl.Acad.Sci.USA, 113, 7792-7797. doi: 10.1073/pnas.1604591113.
- Abstract
- NEIL1 (Nei-like 1) is a DNA repair glycosylase guarding the mammalian genome against oxidized DNA bases. As the first enzymes in the base-excision repair pathway, glycosylases must recognize the cognate substrates and catalyze their excision. Here we present crystal structures of human NEIL1 bound to a range of duplex DNA. Together with computational and biochemical analyses, our results suggest that NEIL1 promotes tautomerization of thymine glycol (Tg)-a preferred substrate-for optimal binding in its active site. Moreover, this tautomerization event also facilitates NEIL1-catalyzed Tg excision. To our knowledge, the present example represents the first documented case of enzyme-promoted tautomerization for efficient substrate recognition and catalysis in an enzyme-catalyzed reaction.