Summary information and primary citation
- PDB-id
- 5iud; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase-DNA
- Method
- X-ray (3.3 Å)
- Summary
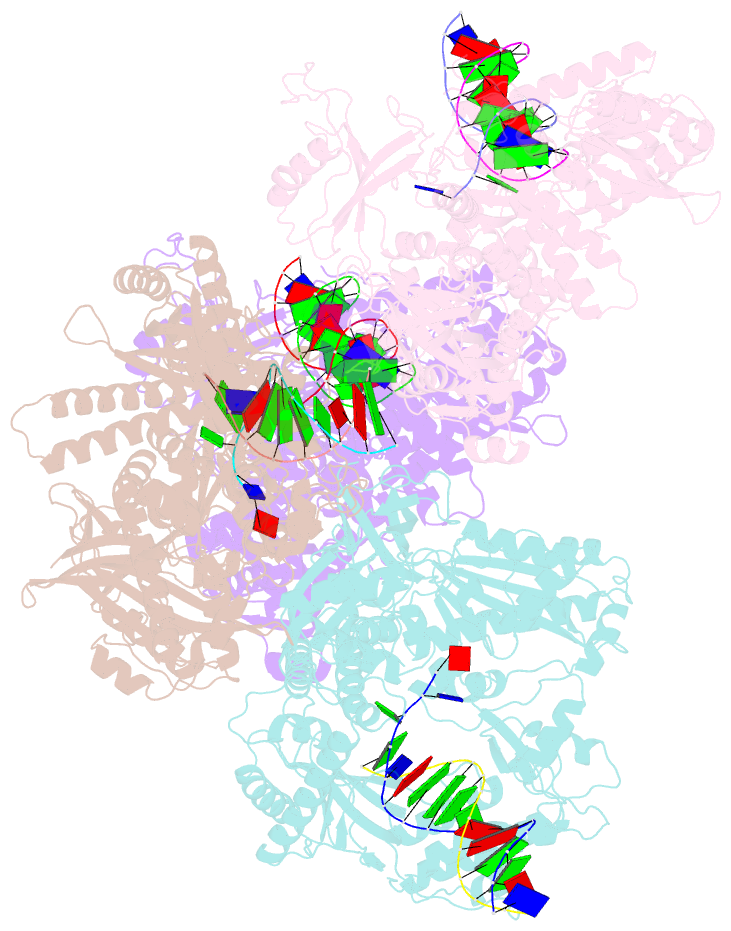
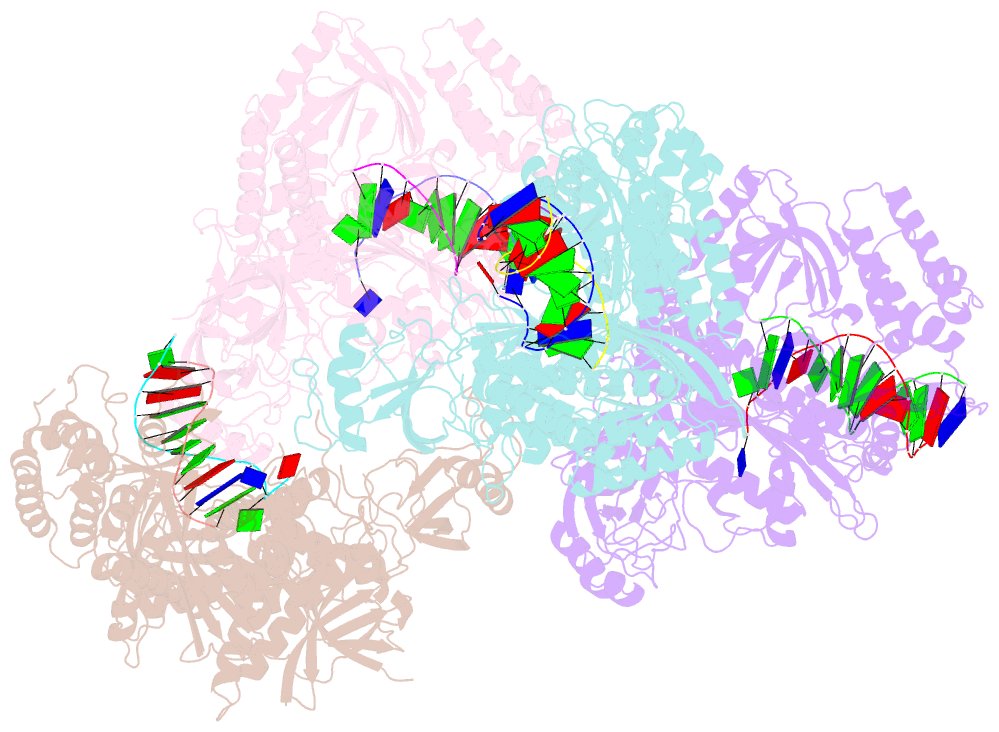
- Human DNA polymerase alpha in binary complex with a DNA:DNA template-primer
- Reference
- Coloma J, Johnson RE, Prakash L, Prakash S, Aggarwal AK (2016): "Human DNA polymerase alpha in binary complex with a DNA:DNA template-primer." Sci Rep, 6, 23784. doi: 10.1038/srep23784.
- Abstract
- The Polα/primase complex assembles the short RNA-DNA fragments for priming of lagging and leading strand DNA replication in eukaryotes. As such, the Polα polymerase subunit encounters two types of substrates during primer synthesis: an RNA:DNA helix and a DNA:DNA helix. The engagement of the polymerase subunit with the DNA:DNA helix has been suggested as the of basis for primer termination in eukaryotes. However, there is no structural information on how the Polα polymerase subunit actually engages with a DNA:DNA helix during primer synthesis. We present here the first crystal structure of human Polα polymerase subunit in complex with a DNA:DNA helix. Unexpectedly, we find that portion of the DNA:DNA helix in contact with the polymerase is not in a B-form but in a hybrid A-B form. Almost all of the contacts observed previously with an RNA primer are preserved with a DNA primer--with the same set of polymerase residues tracking the sugar-phosphate backbone of the DNA or RNA primer. Thus, rather than loss of specific contacts, the free energy cost of distorting DNA from B- to hybrid A-B form may augur the termination of primer synthesis in eukaryotes.