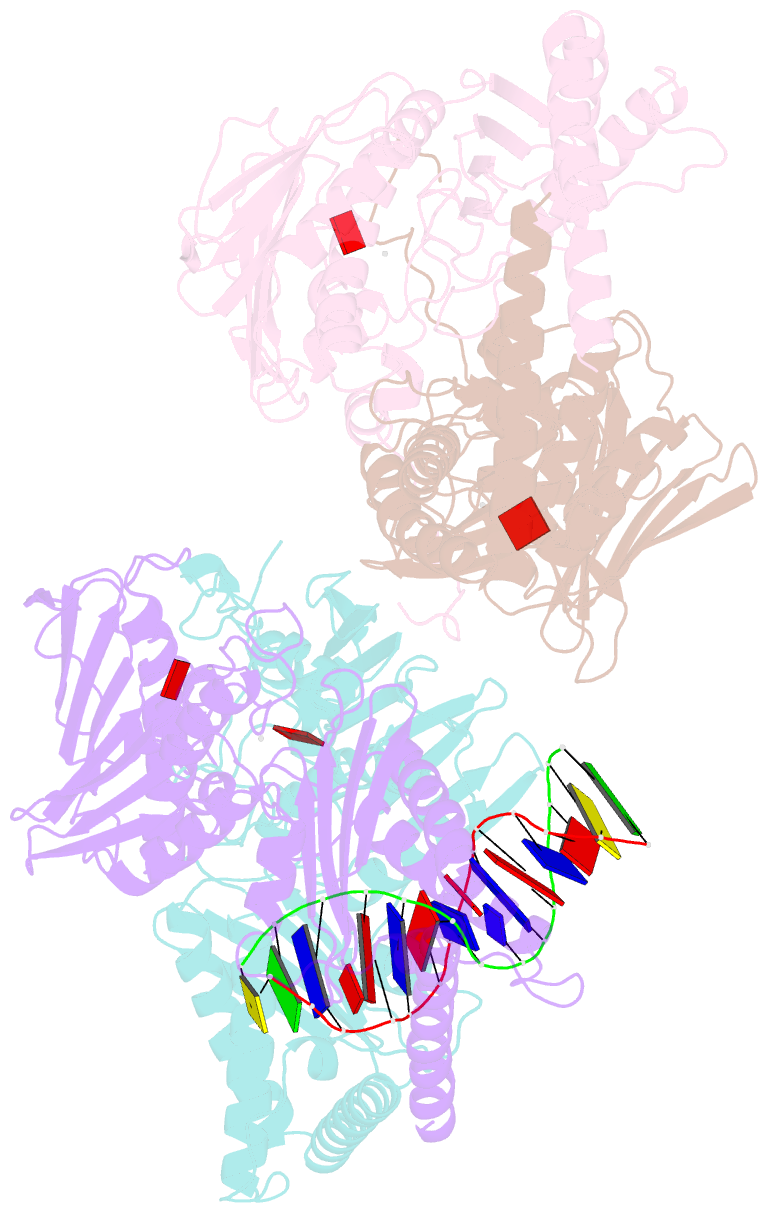
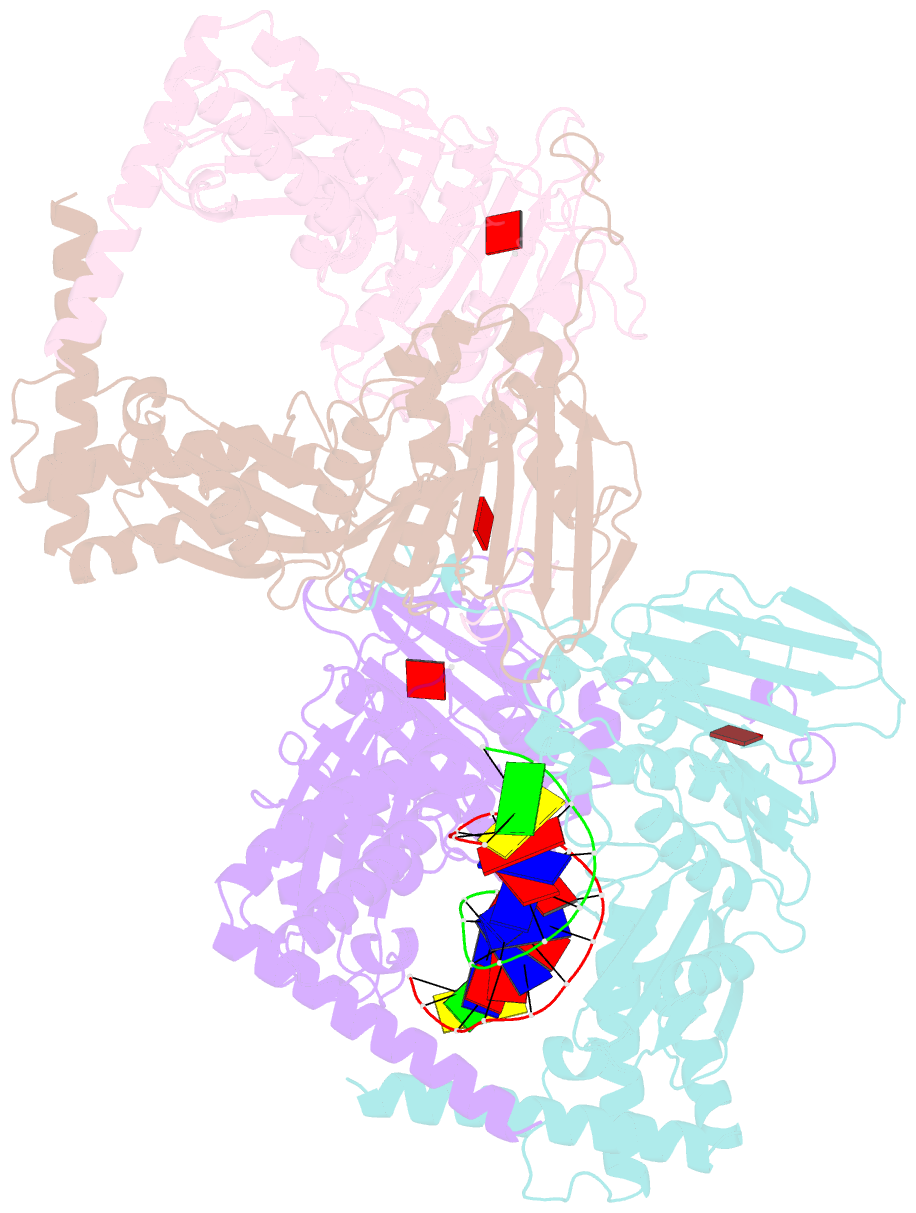
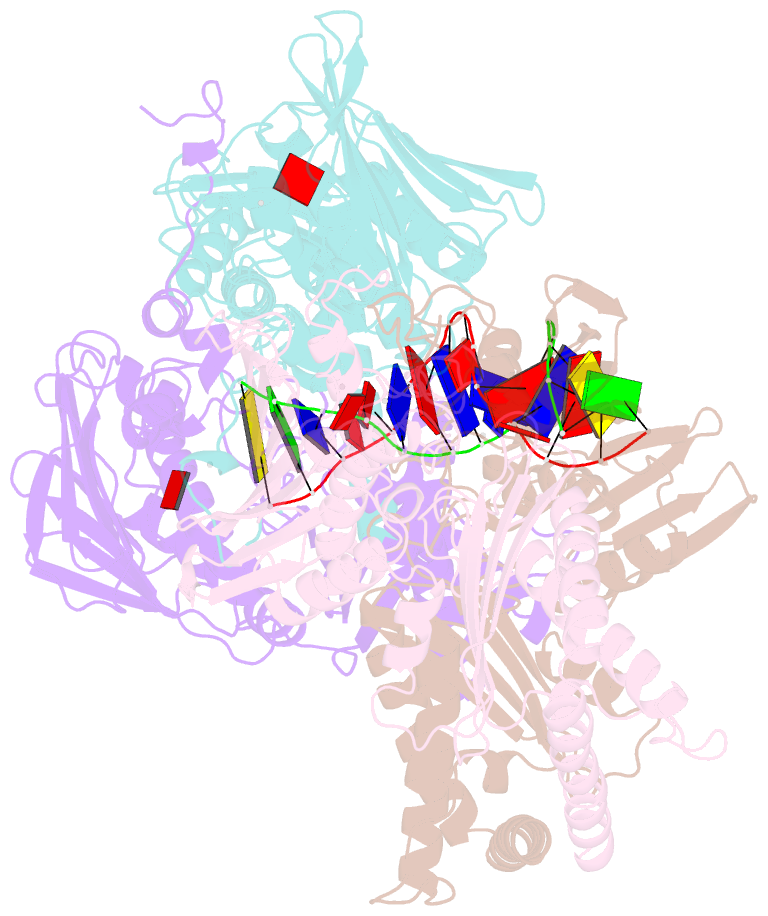
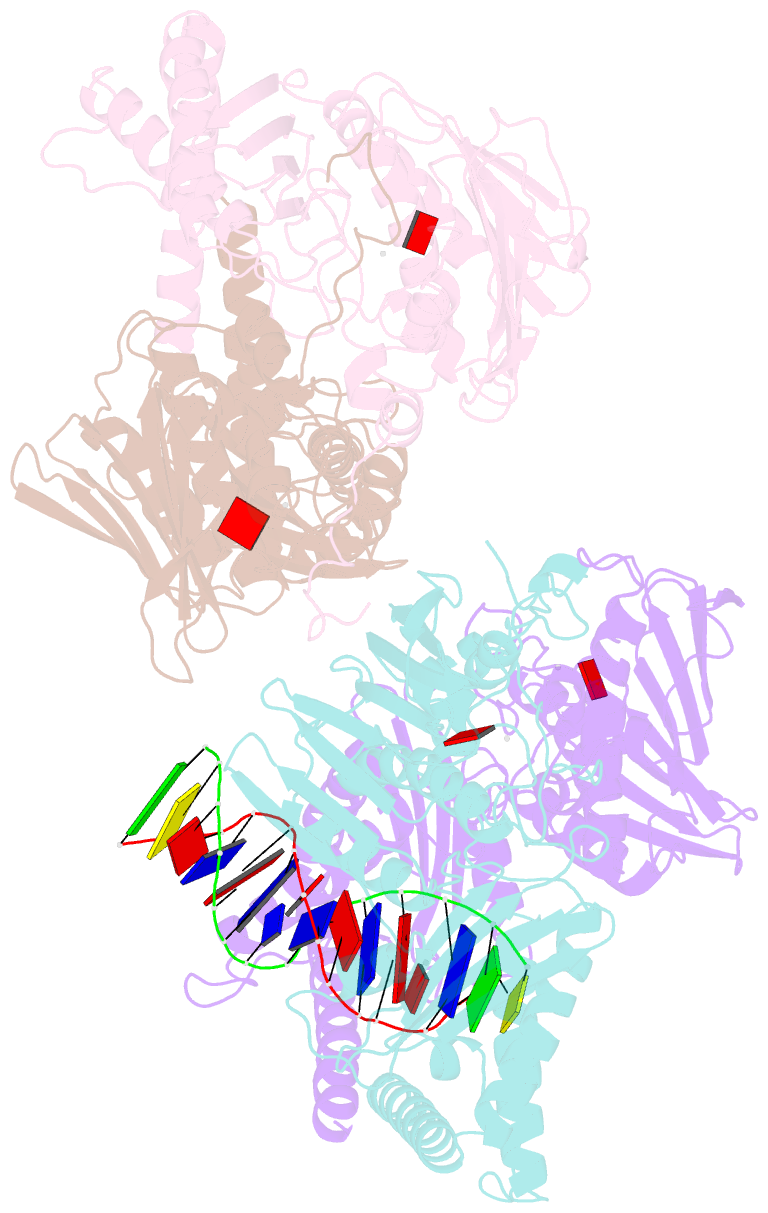
Summary information and primary citation
- PDB-id
- 5j5q; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- isomerase-DNA
- Method
- X-ray (2.83 Å)
- Summary
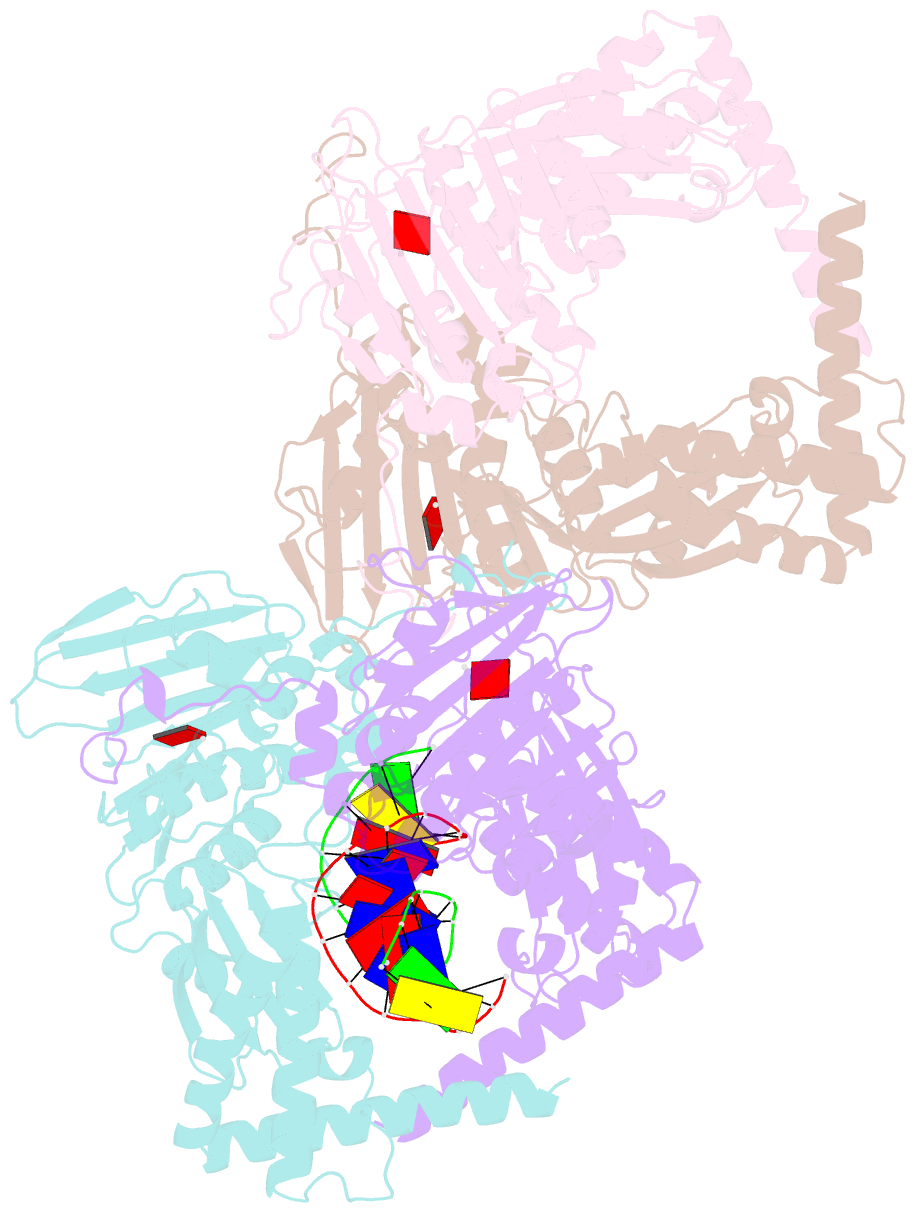
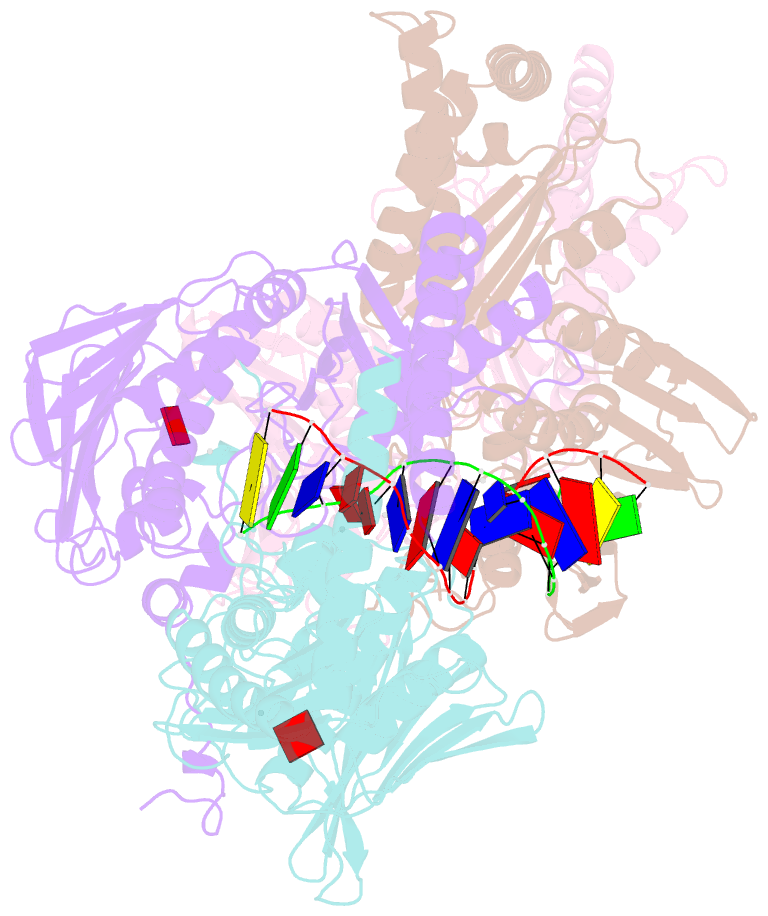
- Amp-pnp-stabilized atpase domain of topoisomerase iv from streptococcus pneumoniae, complex type ii
- Reference
- Laponogov I, Pan XS, Veselkov DA, Skamrova GB, Umrekar TR, Fisher LM, Sanderson MR (2018): "Trapping of the transport-segment DNA by the ATPase domains of a type II topoisomerase." Nat Commun, 9, 2579. doi: 10.1038/s41467-018-05005-x.
- Abstract
- Type II topoisomerases alter DNA topology to control DNA supercoiling and chromosome segregation and are targets of clinically important anti-infective and anticancer therapeutics. They act as ATP-operated clamps to trap a DNA helix and transport it through a transient break in a second DNA. Here, we present the first X-ray crystal structure solved at 2.83 Å of a closed clamp complete with trapped T-segment DNA obtained by co-crystallizing the ATPase domain of S. pneumoniae topoisomerase IV with a nonhydrolyzable ATP analogue and 14-mer duplex DNA. The ATPase dimer forms a 22 Å protein hole occupied by the kinked DNA bound asymmetrically through positively charged residues lining the hole, and whose mutagenesis impacts the DNA decatenation, DNA relaxation and DNA-dependent ATPase activities of topo IV. These results and a side-bound DNA-ParE structure help explain how the T-segment DNA is captured and transported by a type II topoisomerase, and reveal a new enzyme-DNA interface for drug discovery.