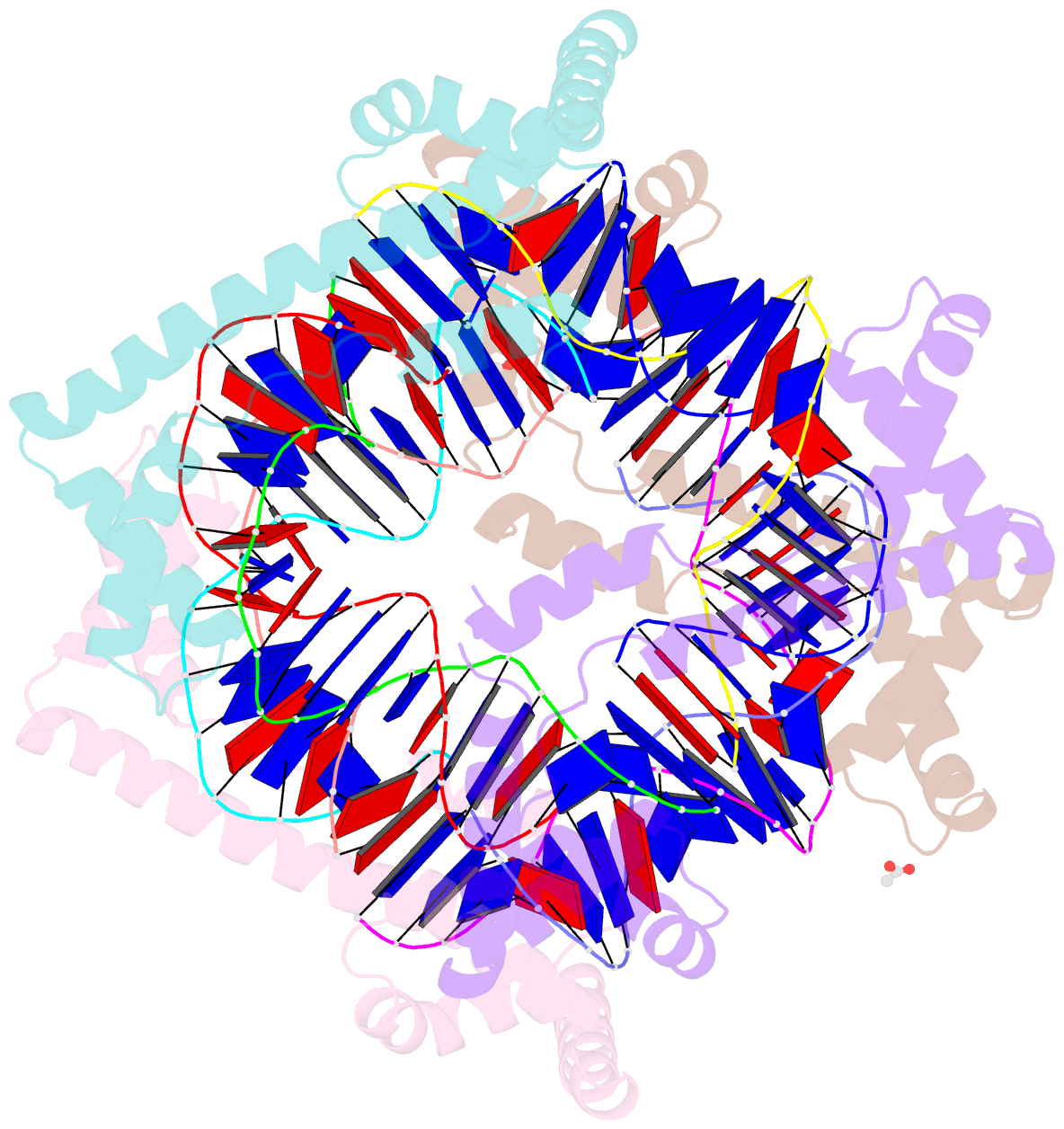
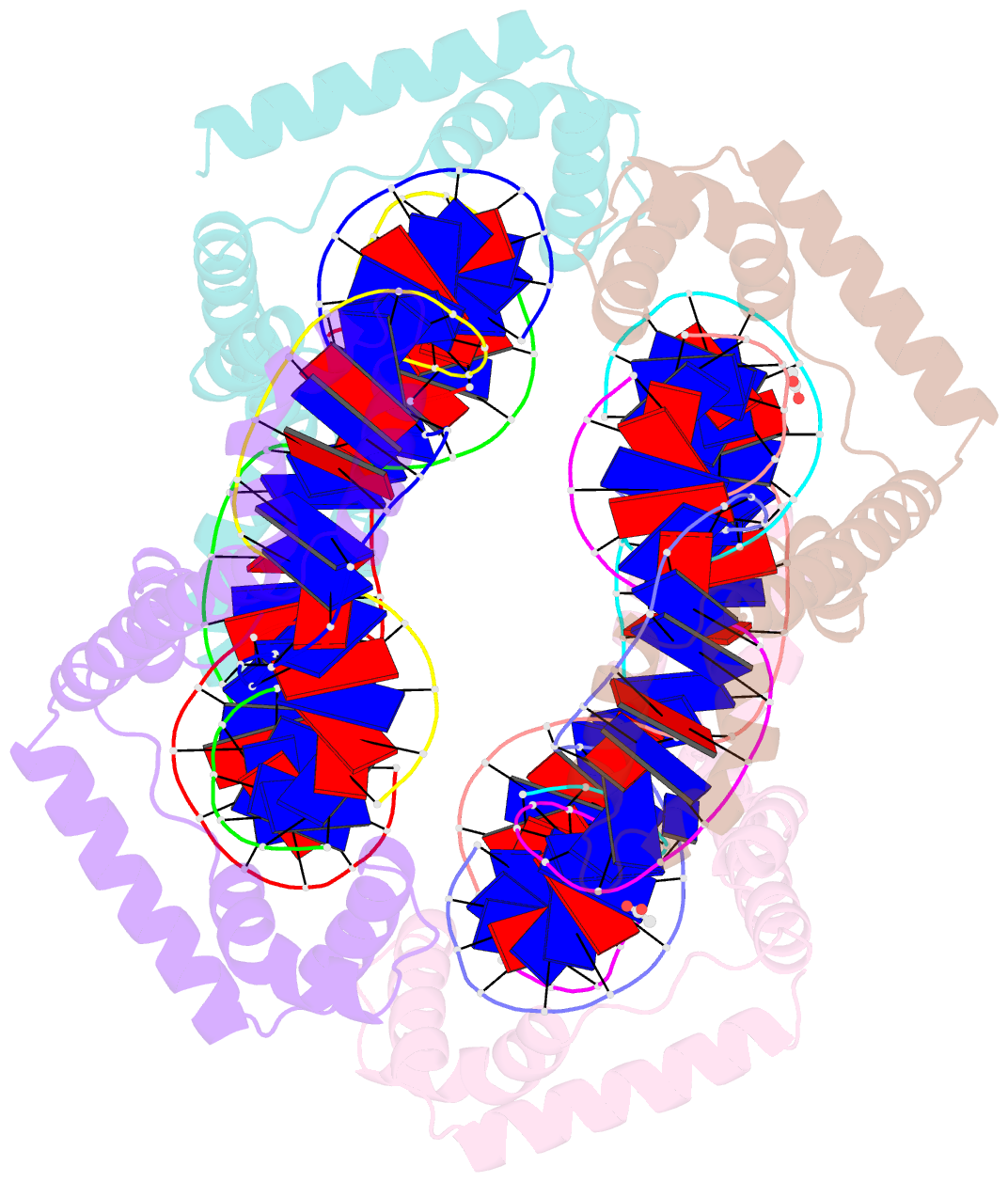
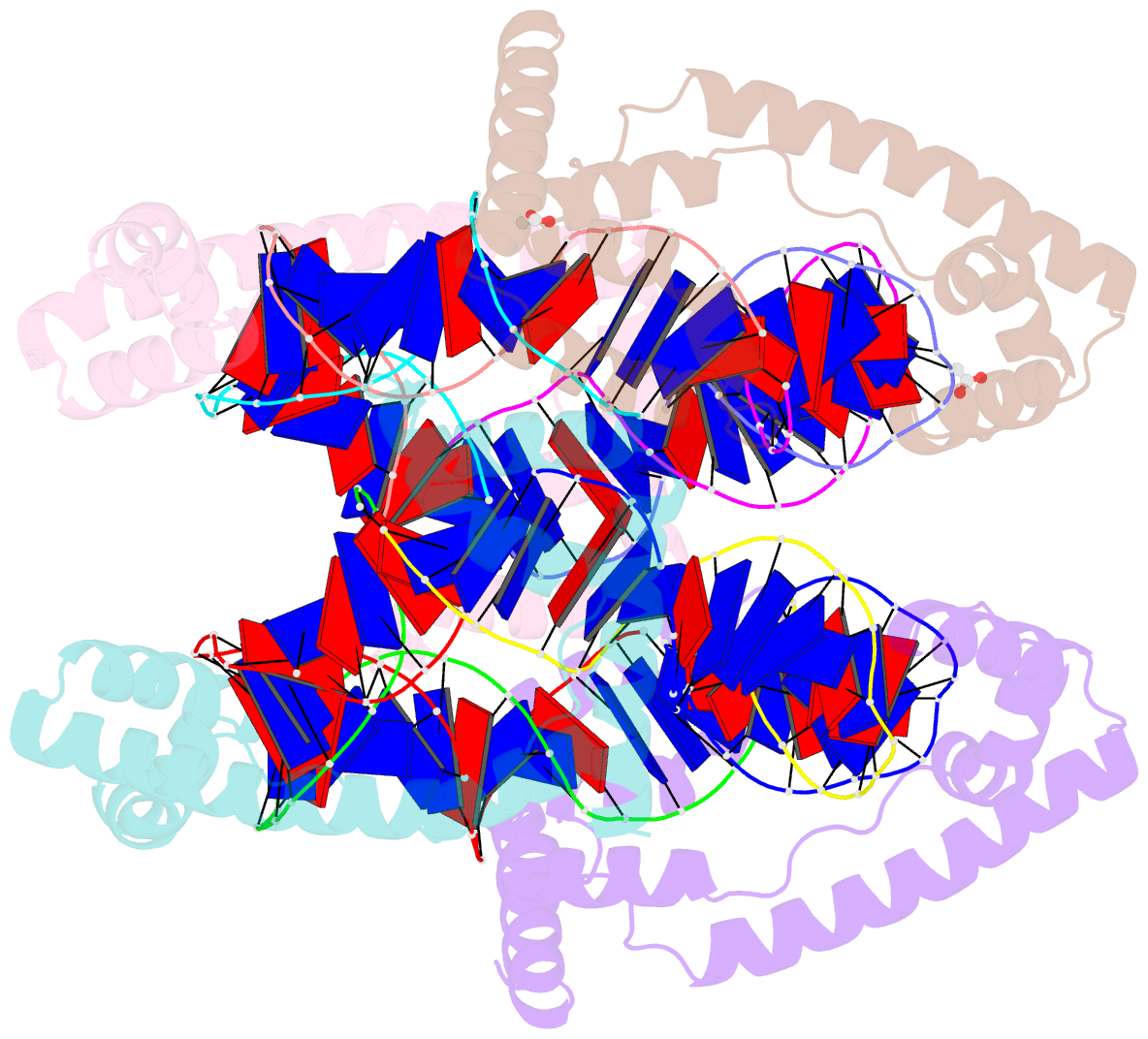
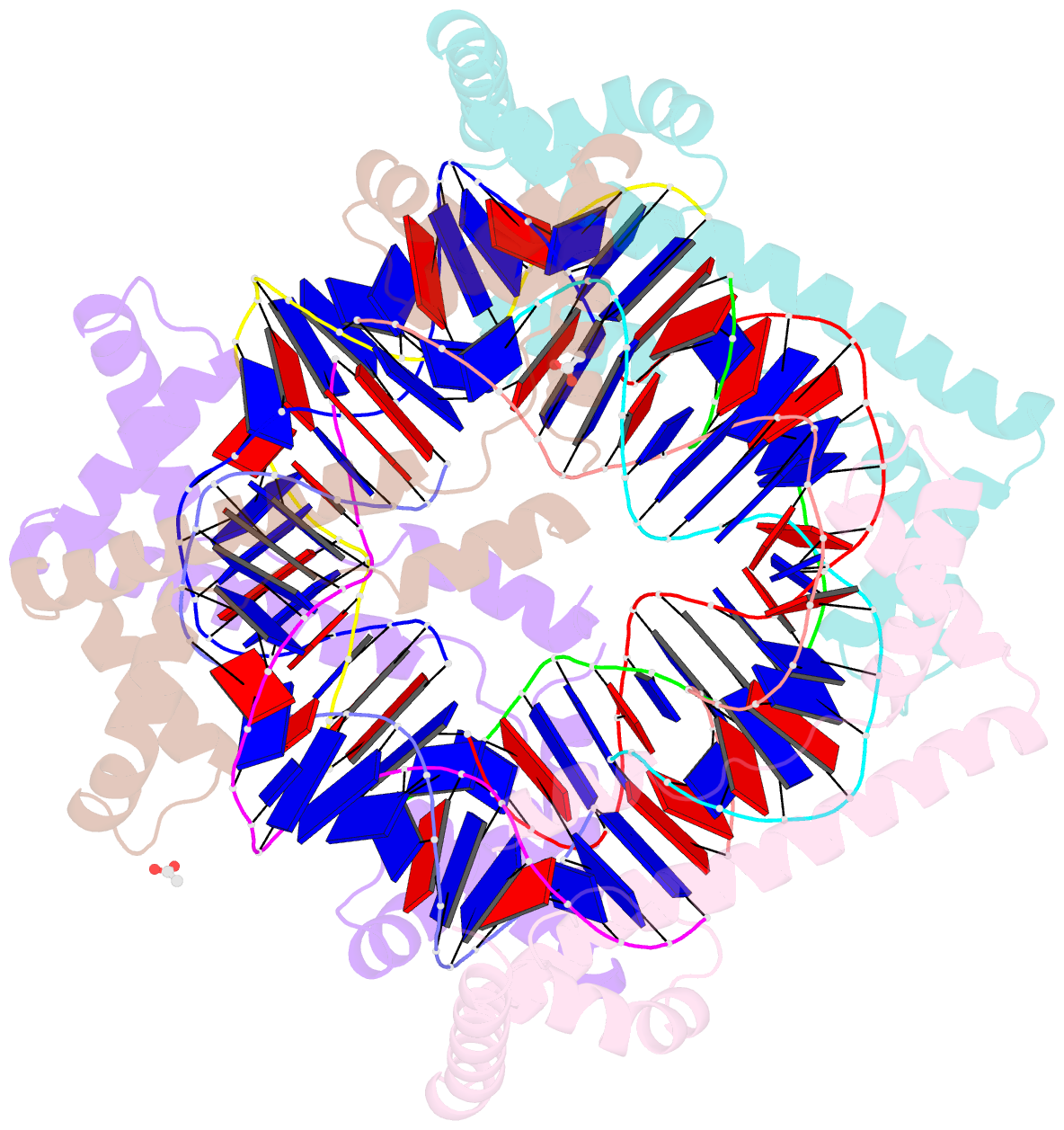
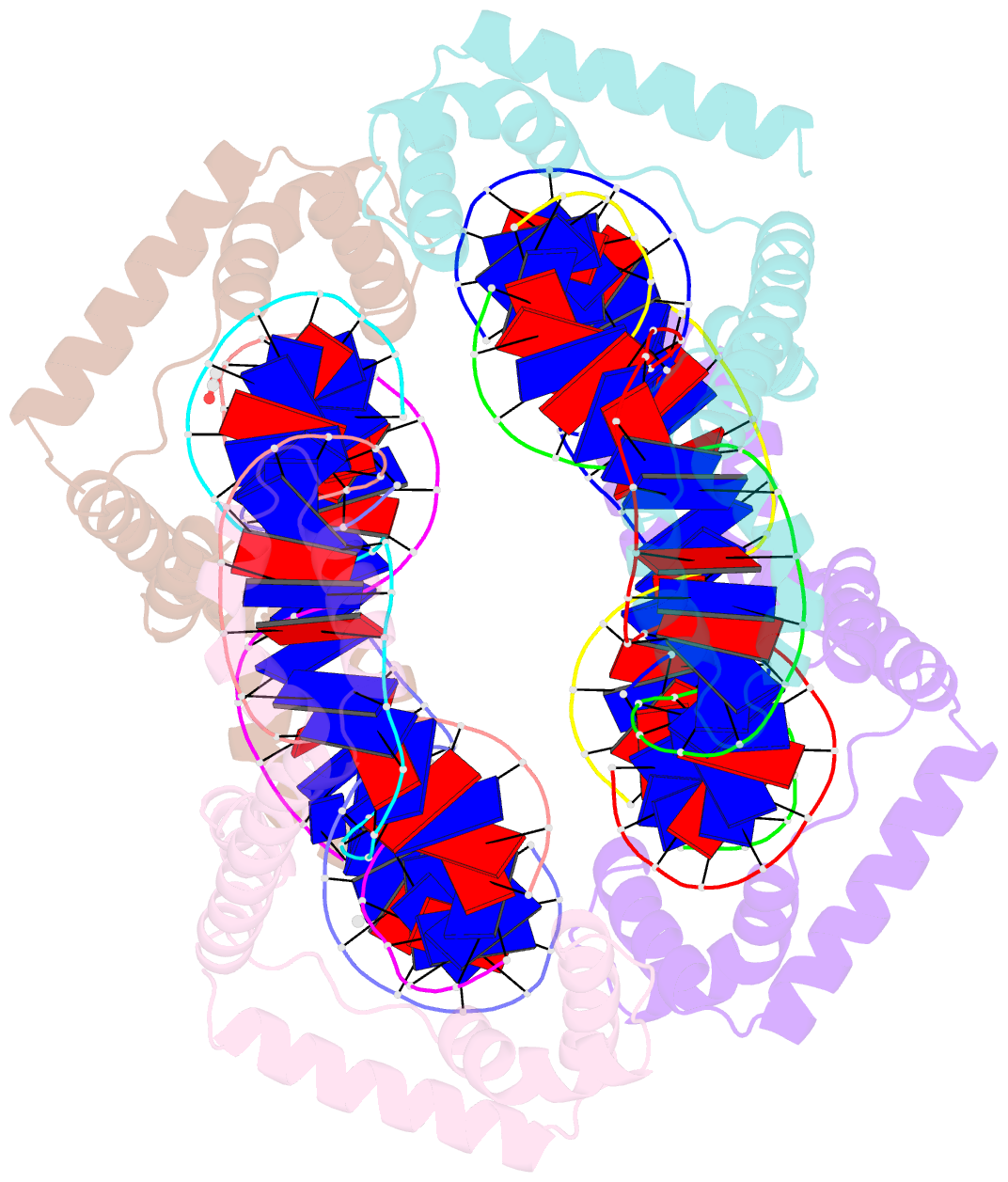
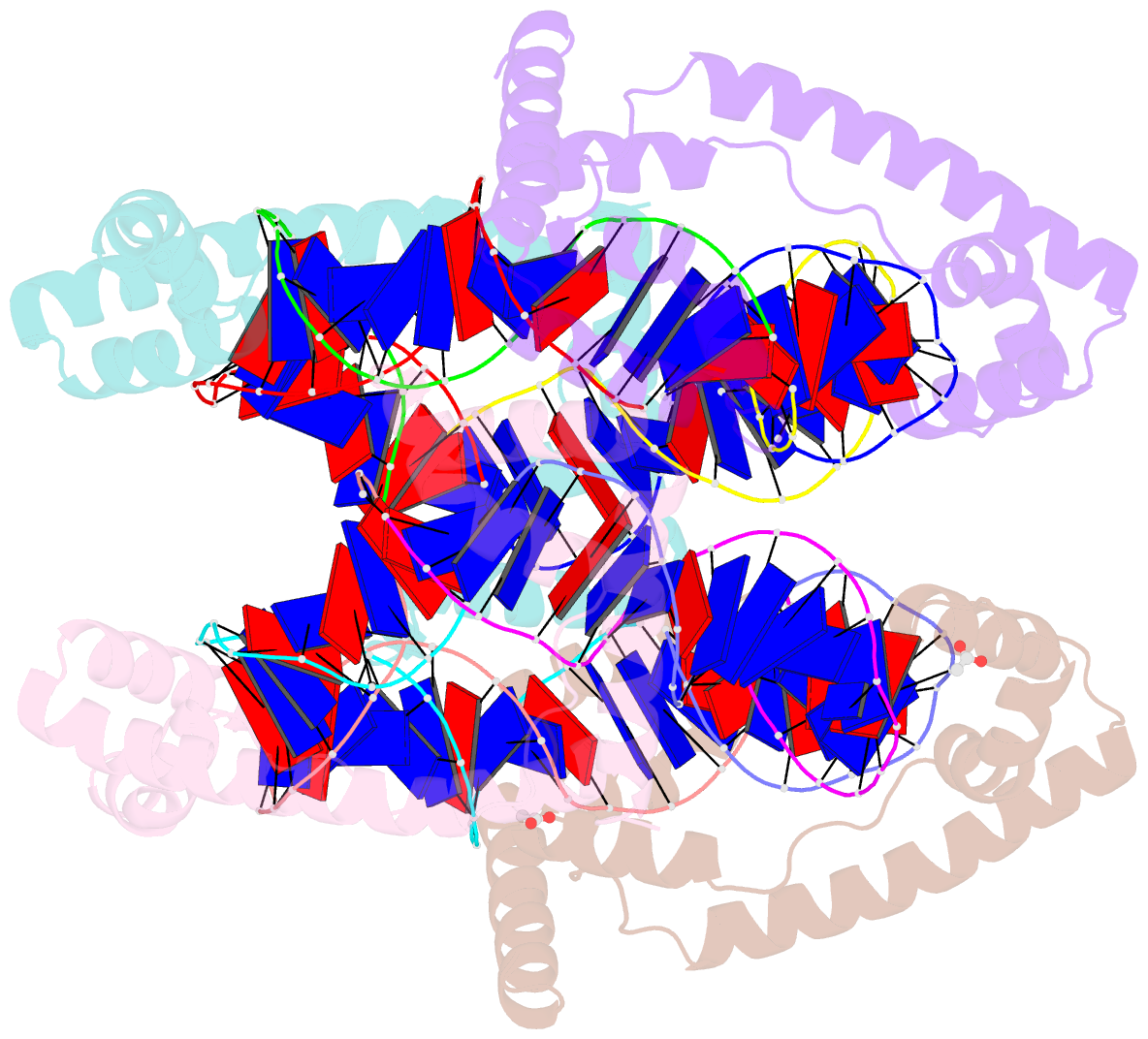
Summary information and primary citation
- PDB-id
- 5jgh; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein
- Method
- X-ray (2.6 Å)
- Summary
- Crystal structure of the mitochondrial DNA packaging protein abf2p in complex with DNA at 2.6 angstrom resolution
- Reference
- Chakraborty A, Lyonnais S, Battistini F, Hospital A, Medici G, Prohens R, Orozco M, Vilardell J, Sola M (2017): "DNA structure directs positioning of the mitochondrial genome packaging protein Abf2p." Nucleic Acids Res., 45, 951-967. doi: 10.1093/nar/gkw1147.
- Abstract
- The mitochondrial genome (mtDNA) is assembled into nucleo-protein structures termed nucleoids and maintained differently compared to nuclear DNA, the involved molecular basis remaining poorly understood. In yeast (Saccharomyces cerevisiae), mtDNA is a ∼80 kbp linear molecule and Abf2p, a double HMG-box protein, packages and maintains it. The protein binds DNA in a non-sequence-specific manner, but displays a distinct 'phased-binding' at specific DNA sequences containing poly-adenine tracts (A-tracts). We present here two crystal structures of Abf2p in complex with mtDNA-derived fragments bearing A-tracts. Each HMG-box of Abf2p induces a 90° bend in the contacted DNA, causing an overall U-turn. Together with previous data, this suggests that U-turn formation is the universal mechanism underlying mtDNA compaction induced by HMG-box proteins. Combining this structural information with mutational, biophysical and computational analyses, we reveal a unique DNA binding mechanism for Abf2p where a characteristic N-terminal flag and helix are crucial for mtDNA maintenance. Additionally, we provide the molecular basis for A-tract mediated exclusion of Abf2p binding. Due to high prevalence of A-tracts in yeast mtDNA, this has critical relevance for nucleoid architecture. Therefore, an unprecedented A-tract mediated protein positioning mechanism regulates DNA packaging proteins in the mitochondria, and in combination with DNA-bending and U-turn formation, governs mtDNA compaction.