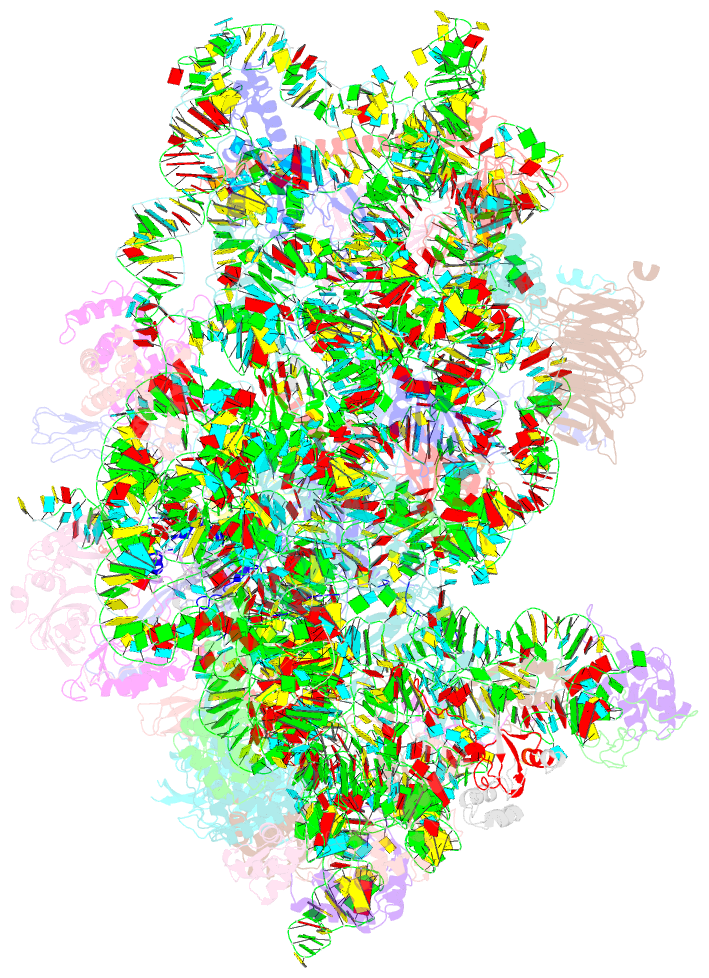
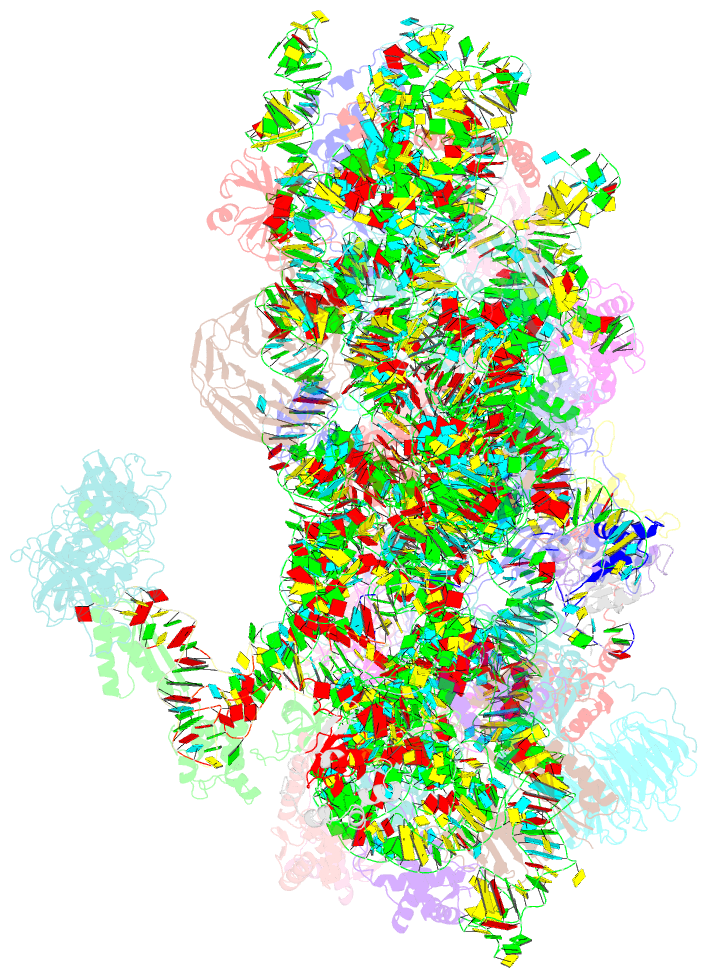
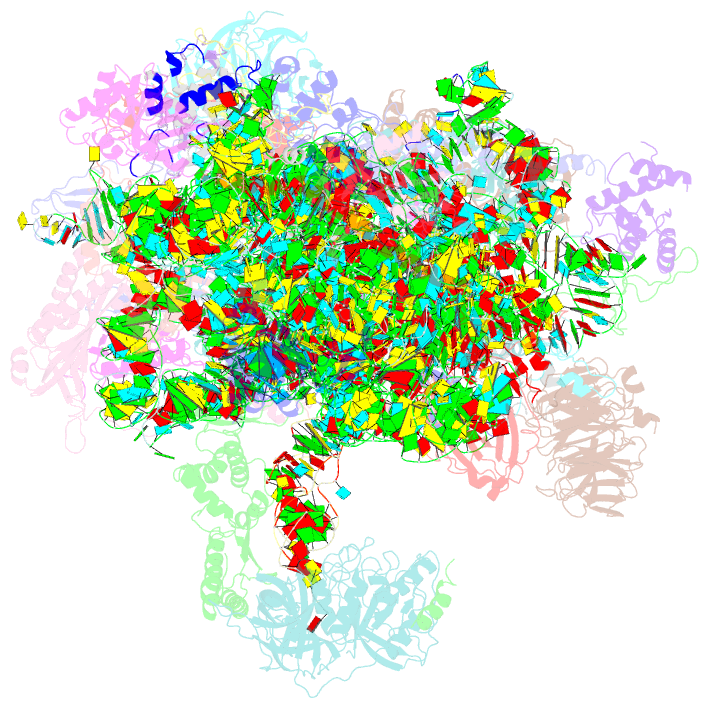
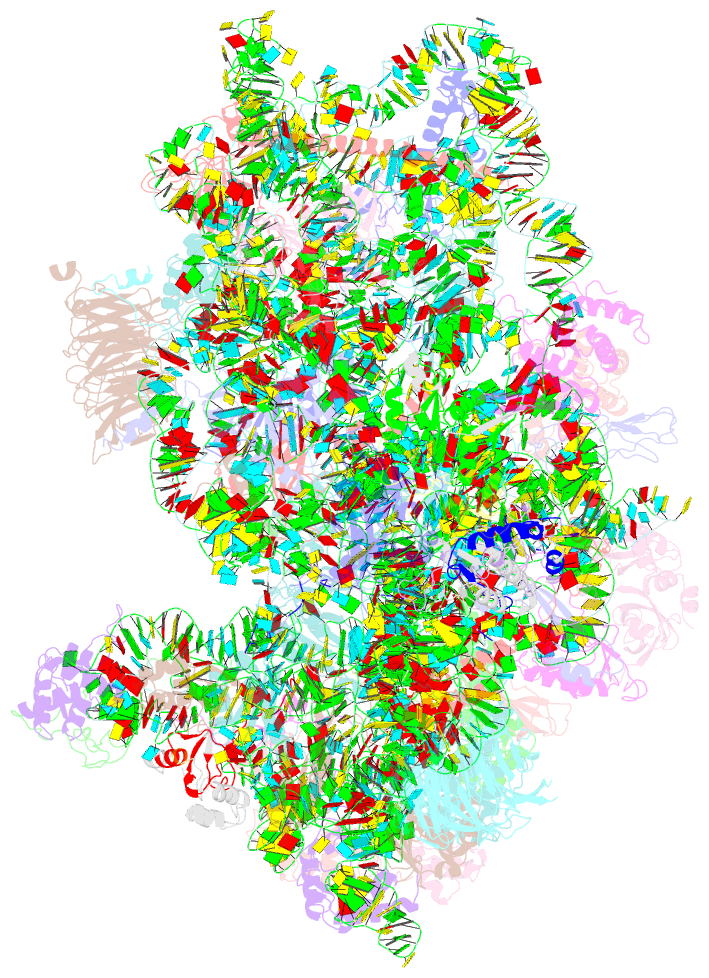
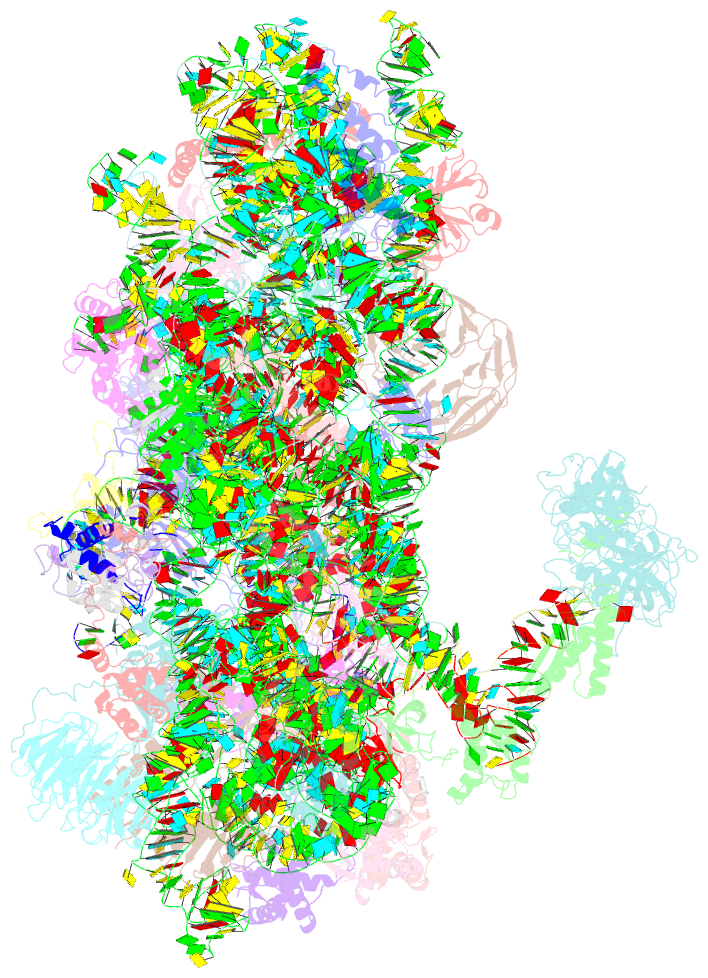
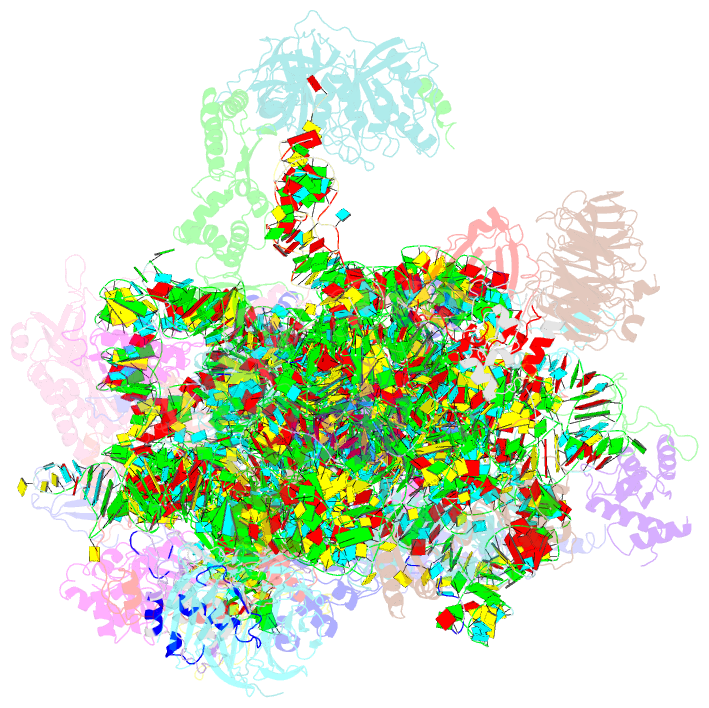
Summary information and primary citation
- PDB-id
- 5k0y; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- translation
- Method
- cryo-EM (5.8 Å)
- Summary
- M48s late-stage initiation complex, purified from rabbit reticulocytes lysates, displaying eif2 ternary complex and eif3 i and g subunits relocated to the intersubunit face
- Reference
- Simonetti A, Brito Querido J, Myasnikov AG, Mancera-Martinez E, Renaud A, Kuhn L, Hashem Y (2016): "eIF3 Peripheral Subunits Rearrangement after mRNA Binding and Start-Codon Recognition." Mol.Cell, 63, 206-217. doi: 10.1016/j.molcel.2016.05.033.
- Abstract
- mRNA translation initiation in eukaryotes requires the cooperation of a dozen eukaryotic initiation factors (eIFs) forming several complexes, which leads to mRNA attachment to the small ribosomal 40S subunit, mRNA scanning for start codon, and accommodation of initiator tRNA at the 40S P site. eIF3, composed of 13 subunits, 8 core (a, c, e, f, h, l, k, and m) and 5 peripheral (b, d, g, i, and j), plays a central role during this process. Here we report a cryo-electron microscopy structure of a mammalian 48S initiation complex at 5.8 Å resolution. It shows the relocation of subunits eIF3i and eIF3g to the 40S intersubunit face on the GTPase binding site, at a late stage in initiation. On the basis of a previous study, we demonstrate the relocation of eIF3b to the 40S intersubunit face, binding below the eIF2-Met-tRNAi(Met) ternary complex upon mRNA attachment. Our analysis reveals the deep rearrangement of eIF3 and unravels the molecular mechanism underlying eIF3 function in mRNA scanning and timing of ribosomal subunit joining.