Summary information and primary citation
- PDB-id
- 5lta; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- protein-RNA
- Method
- X-ray (2.621 Å)
- Summary
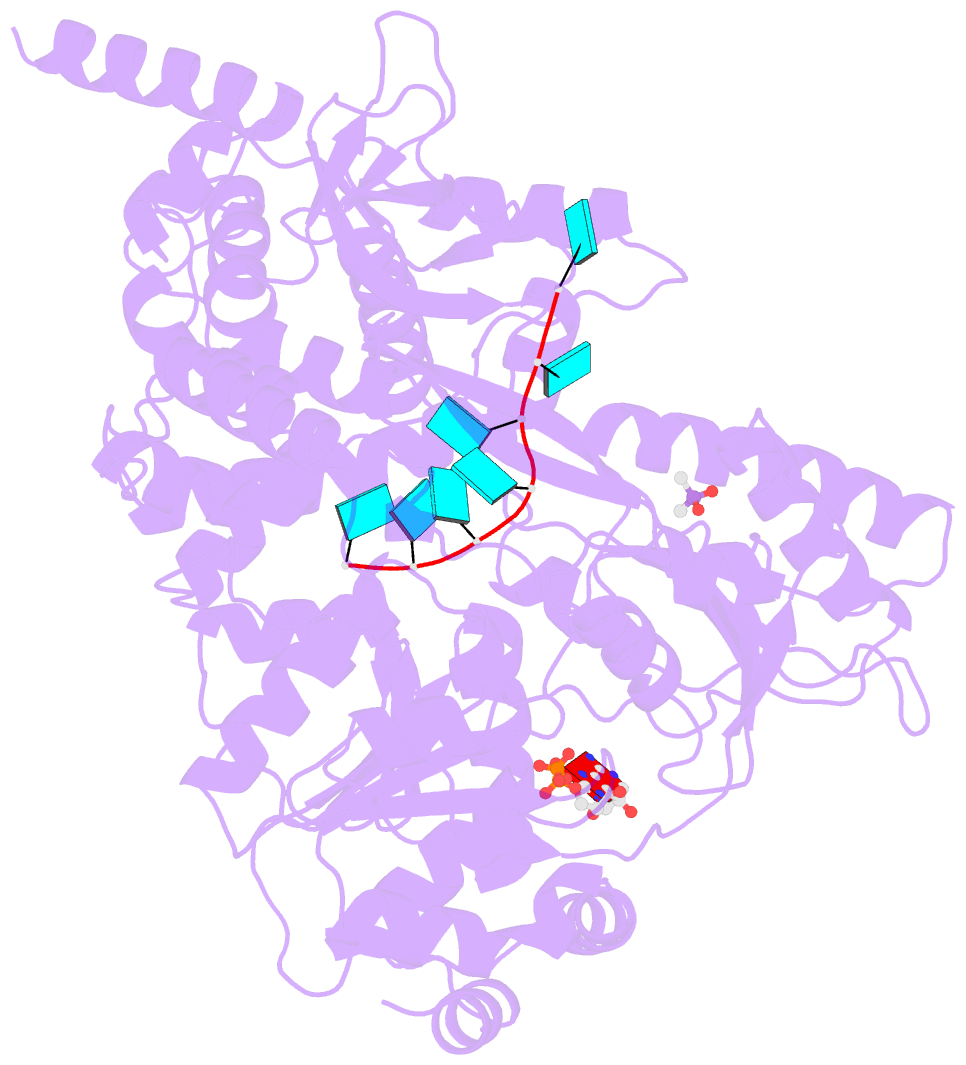
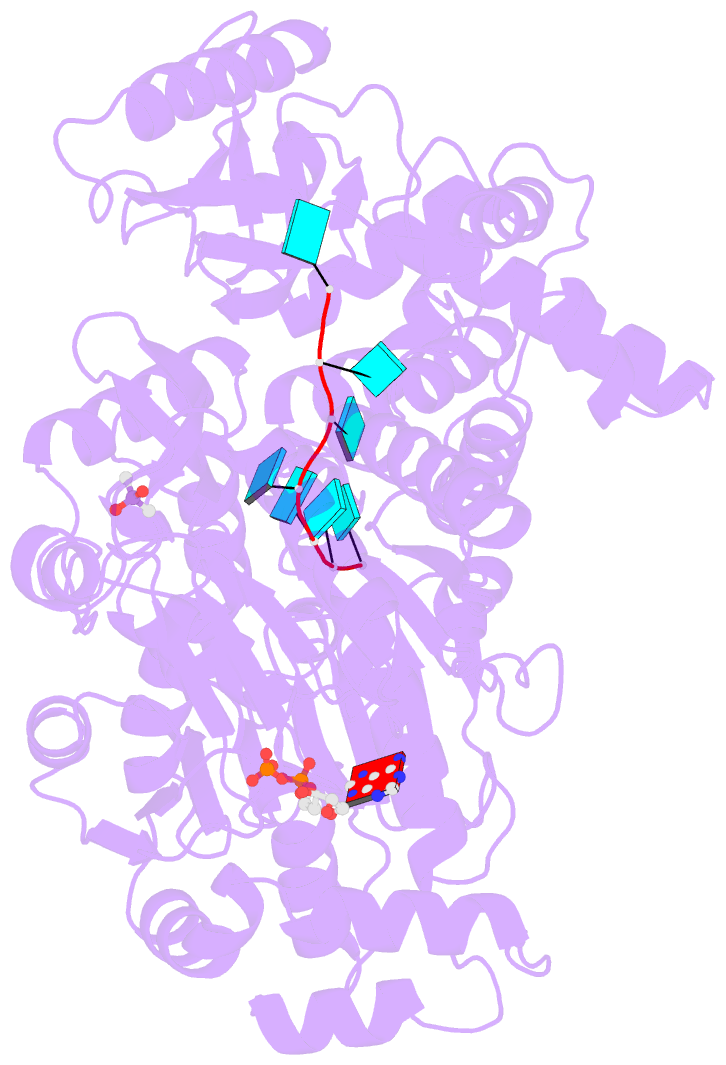
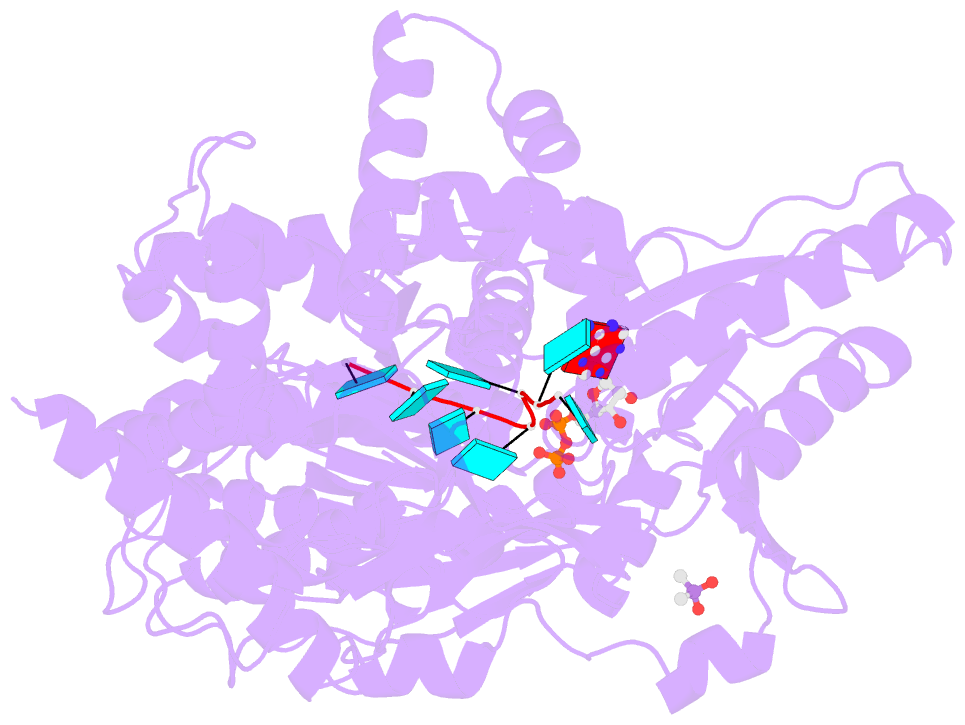
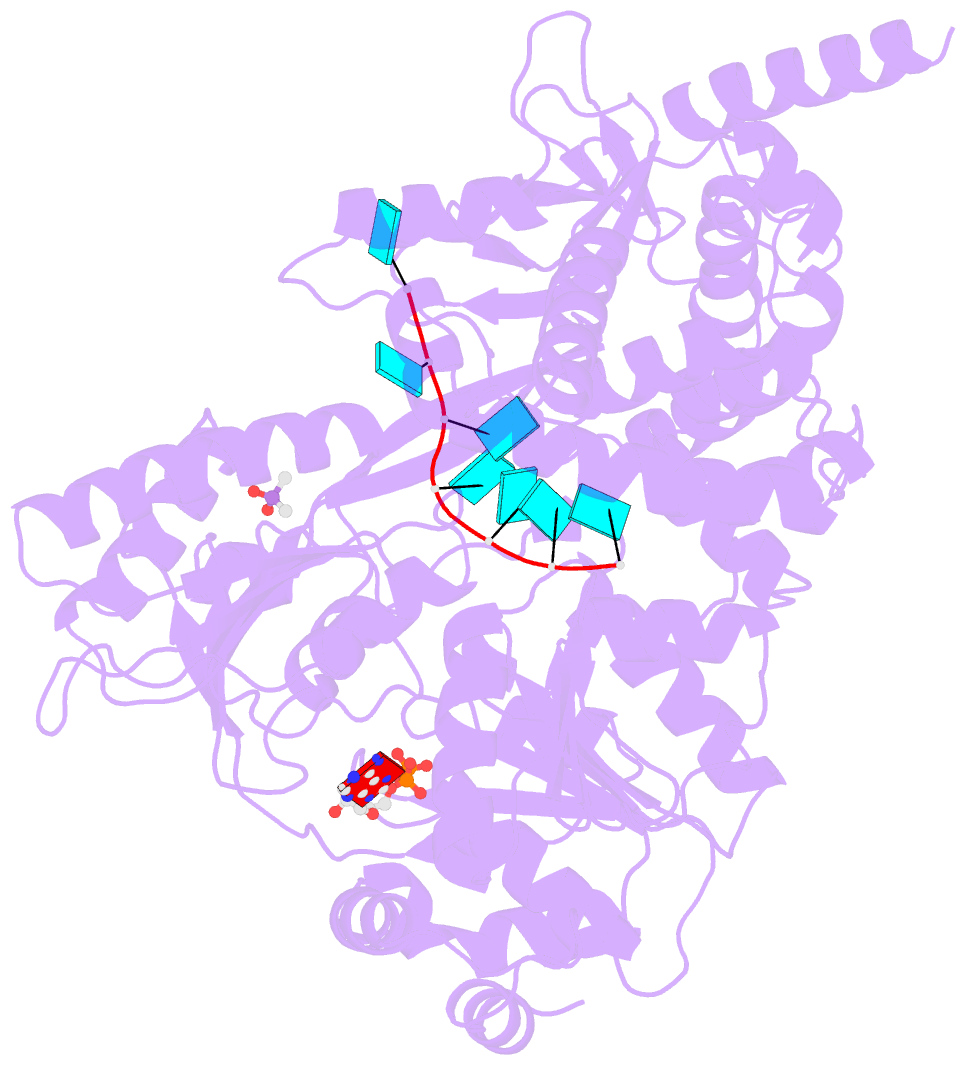
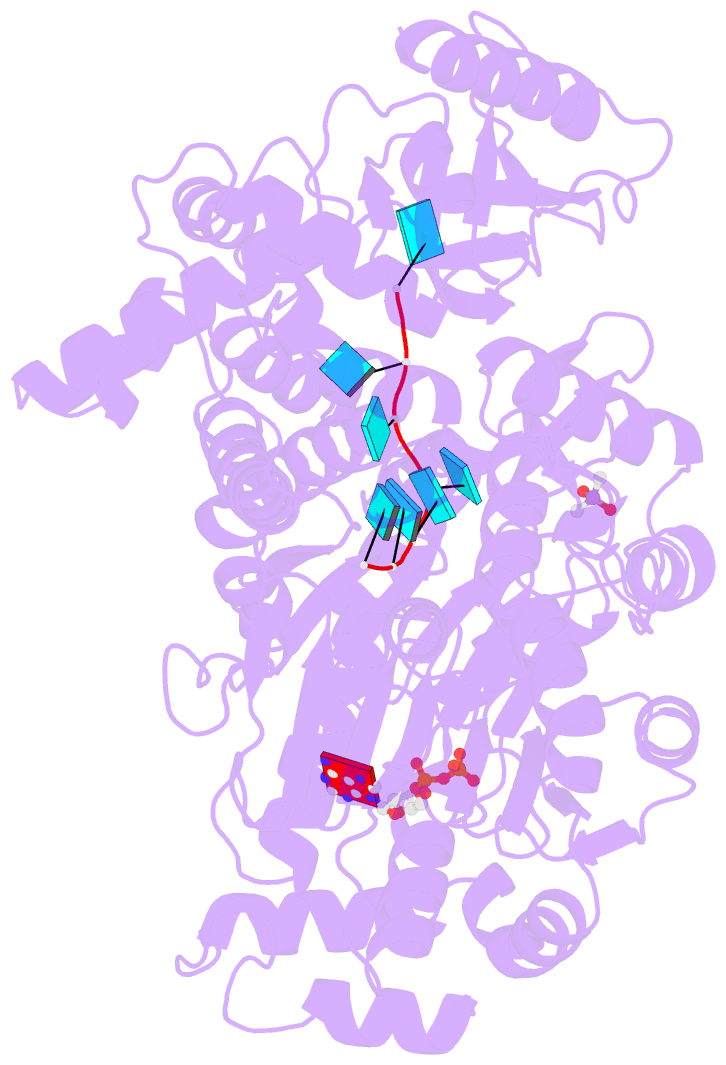
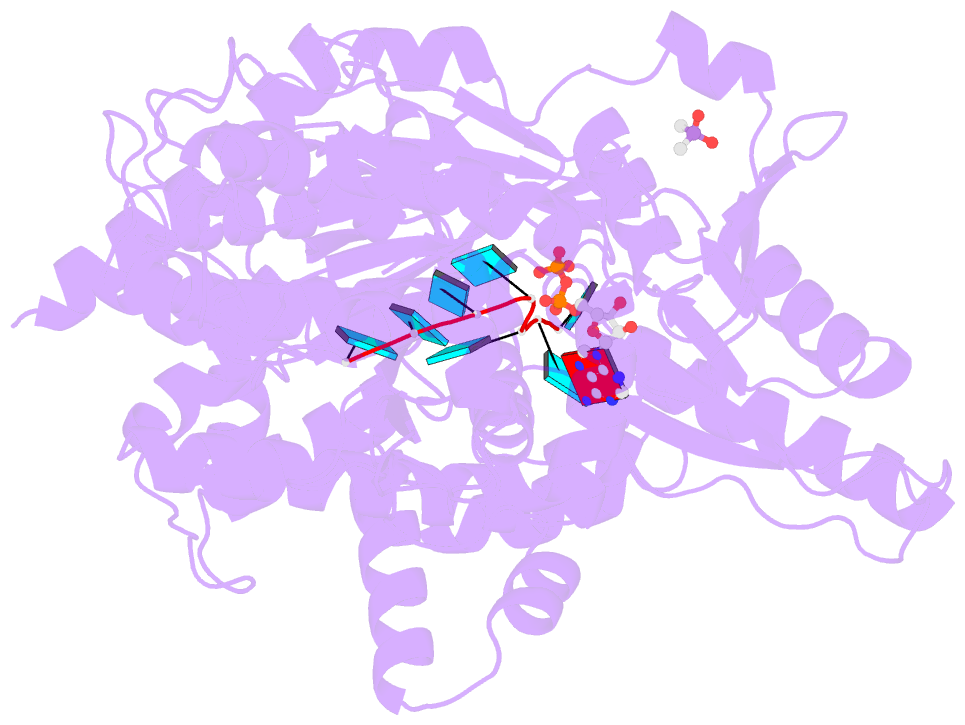
- Crystal structure of the prp43-adp-bef3-u7-RNA complex
- Reference
- Tauchert MJ, Fourmann JB, Luhrmann R, Ficner R (2017): "Structural insights into the mechanism of the DEAH-box RNA helicase Prp43." Elife, 6. doi: 10.7554/eLife.21510.
- Abstract
- The DEAH-box helicase Prp43 is a key player in pre-mRNA splicing as well as the maturation of rRNAs. The exact modus operandi of Prp43 and of all other spliceosomal DEAH-box RNA helicases is still elusive. Here, we report crystal structures of Prp43 complexes in different functional states and the analysis of structure-based mutants providing insights into the unwinding and loading mechanism of RNAs. The Prp43•ATP-analog•RNA complex shows the localization of the RNA inside a tunnel formed by the two RecA-like and C-terminal domains. In the ATP-bound state this tunnel can be transformed into a groove prone for RNA binding by large rearrangements of the C-terminal domains. Several conformational changes between the ATP- and ADP-bound states explain the coupling of ATP hydrolysis to RNA translocation, mainly mediated by a β-turn of the RecA1 domain containing the newly identified RF motif. This mechanism is clearly different to those of other RNA helicases.