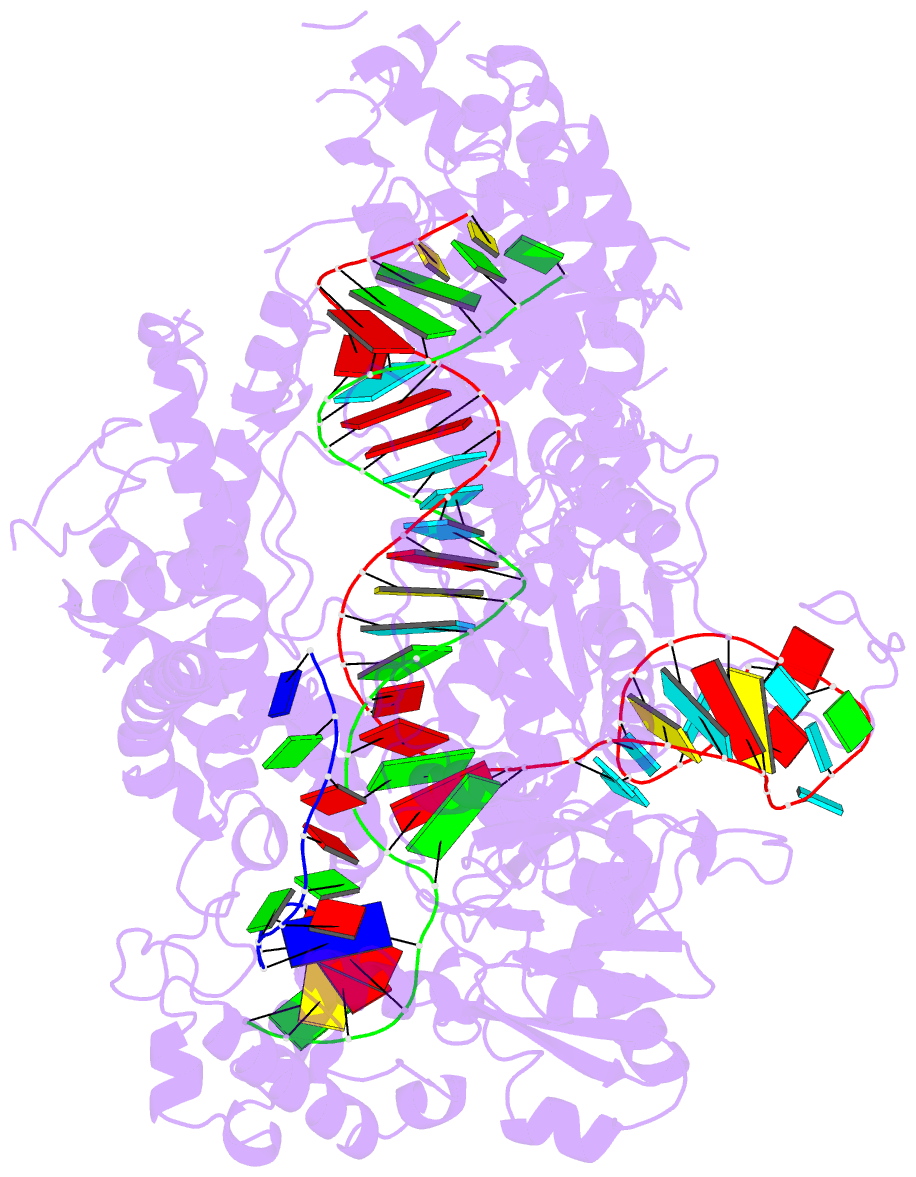
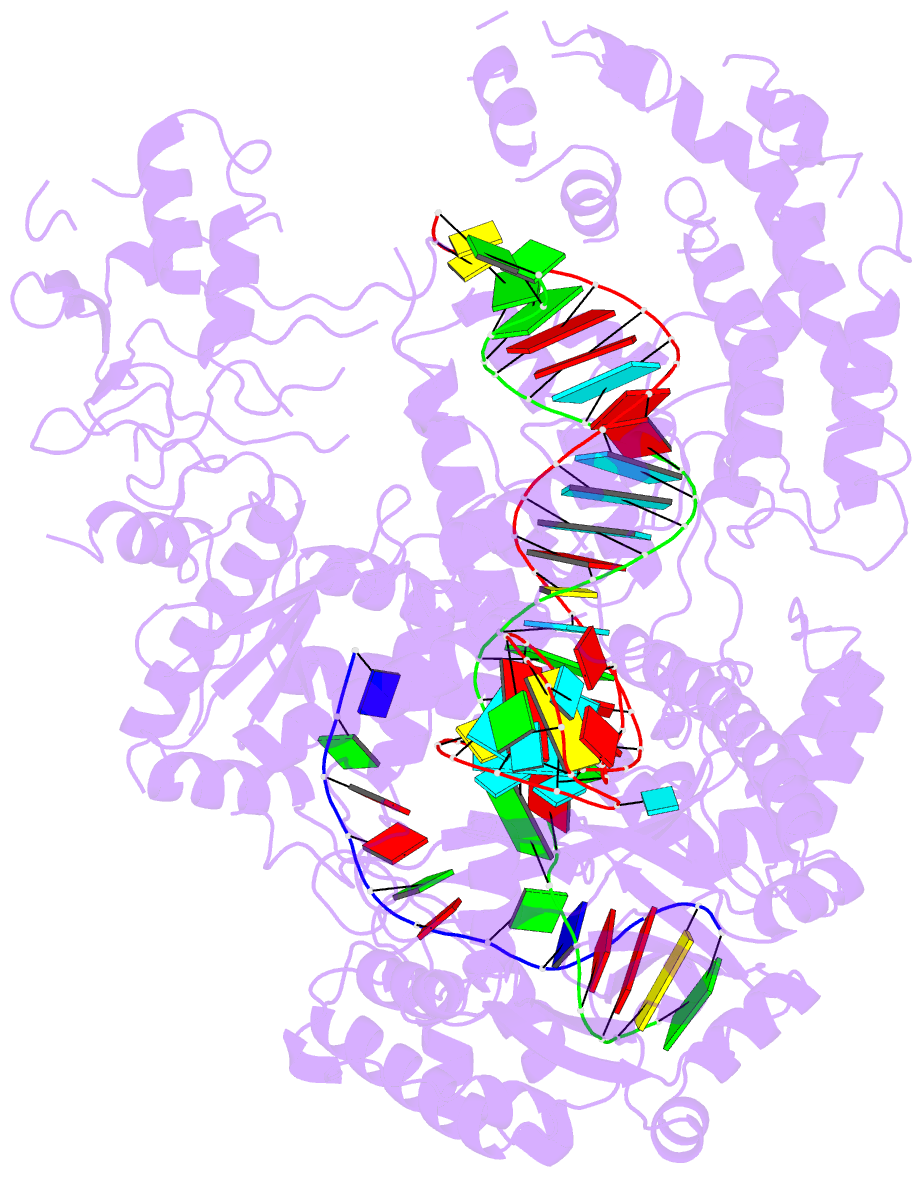
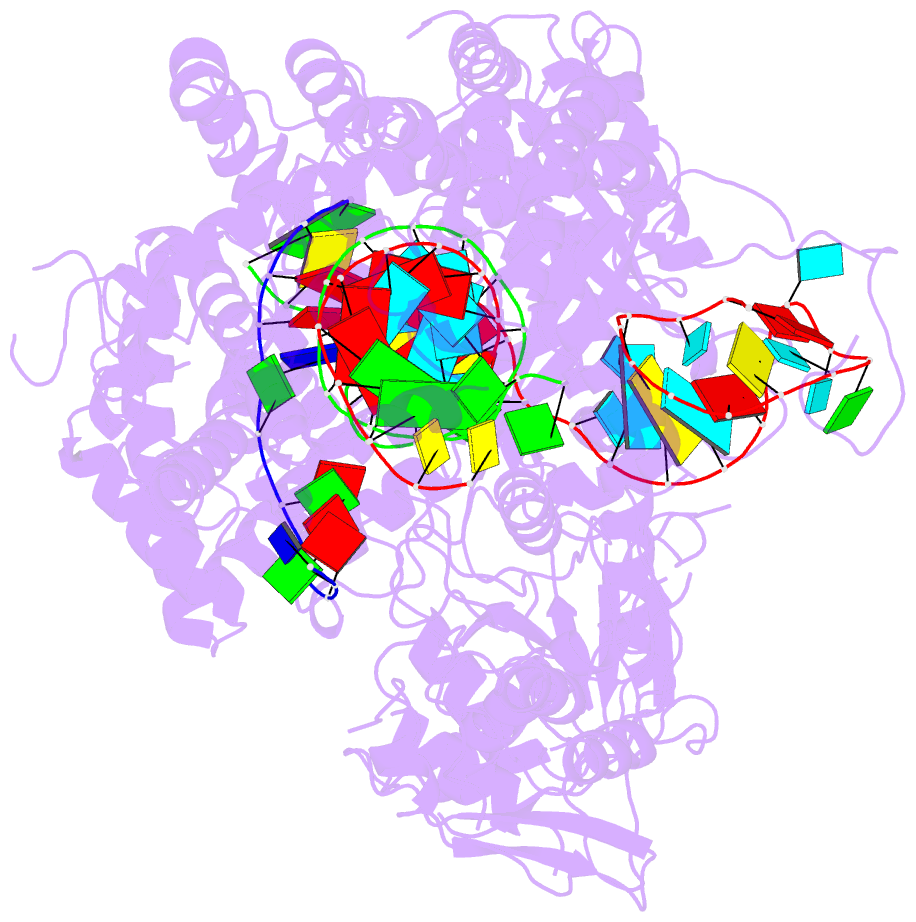
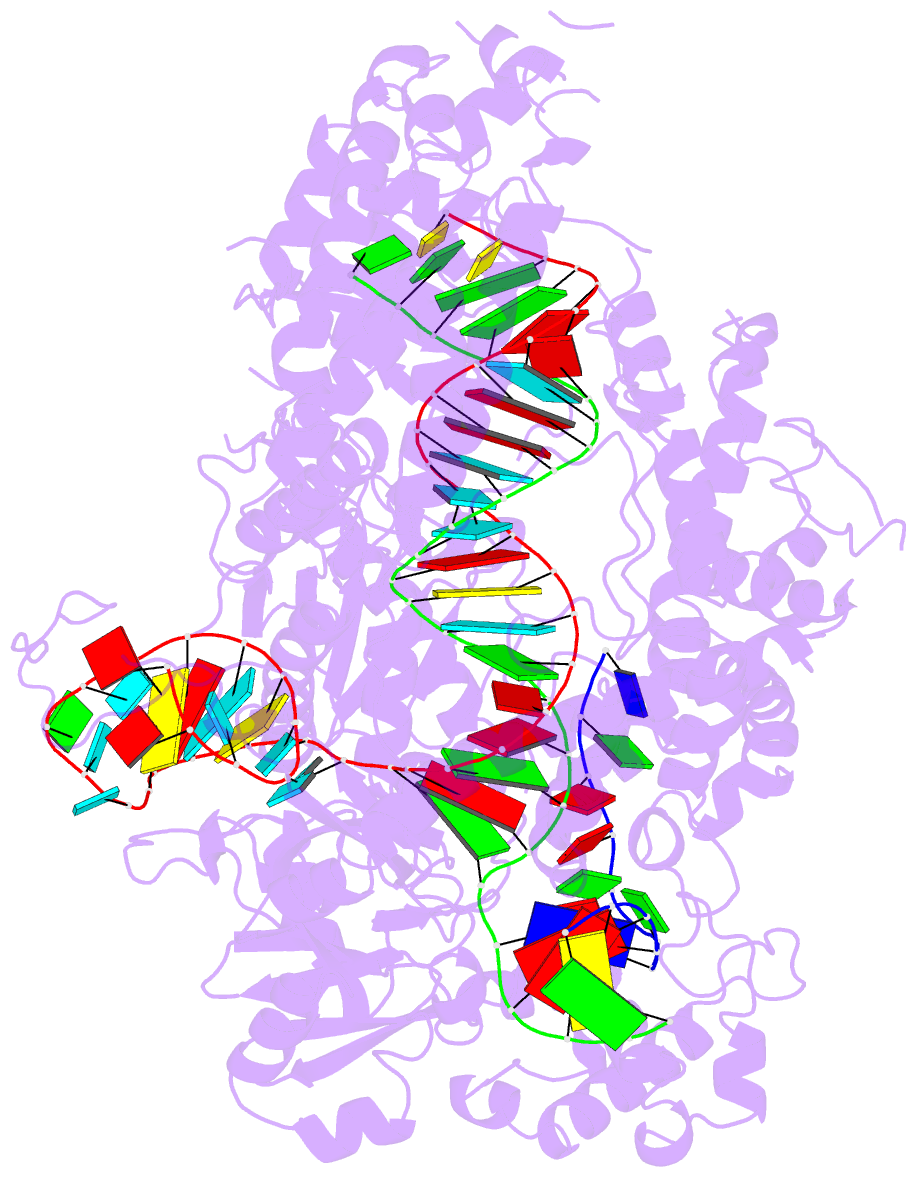
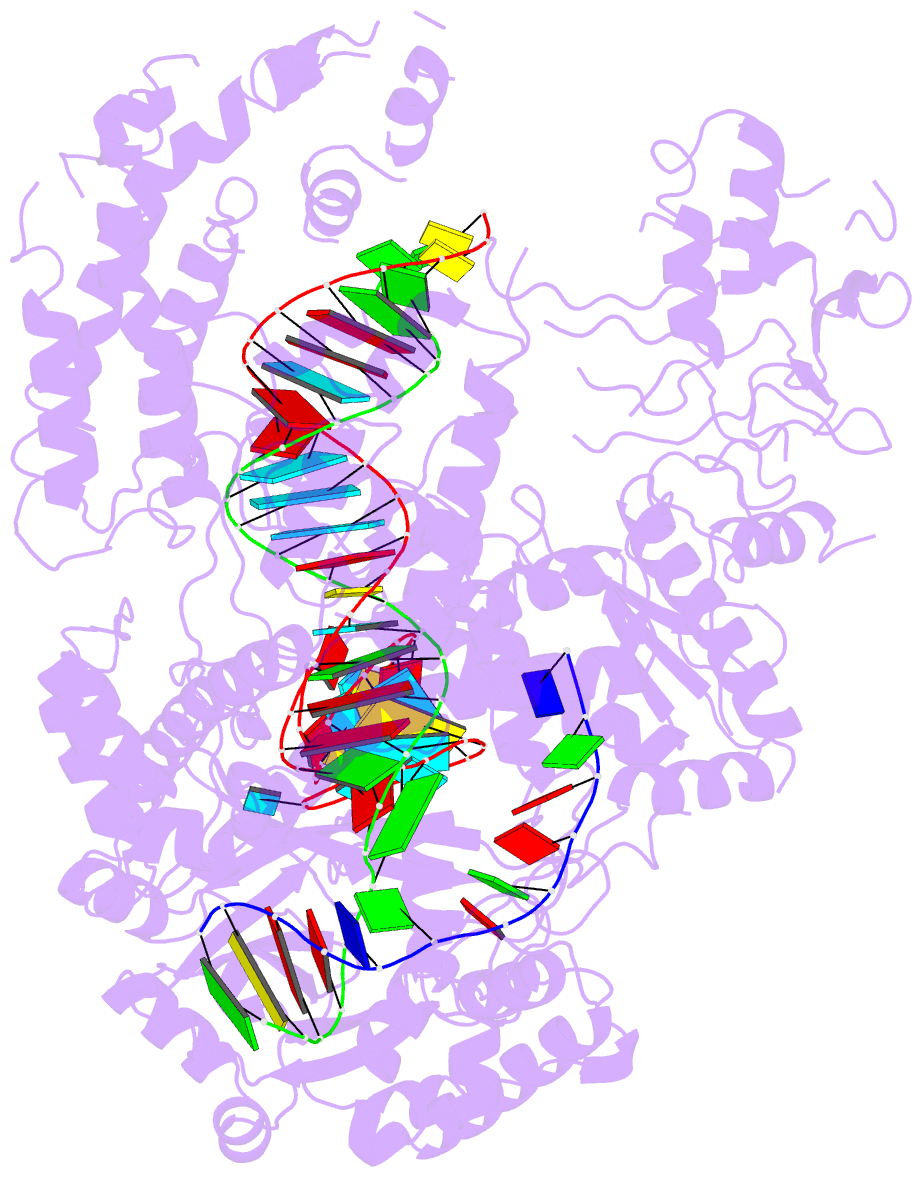
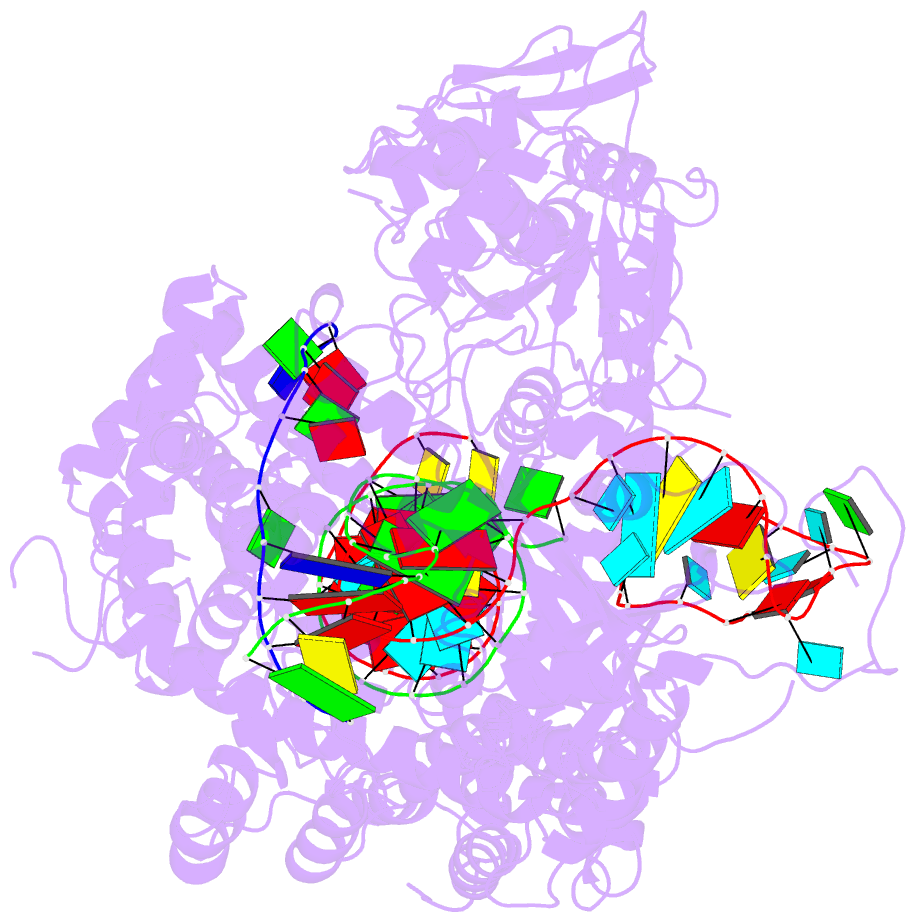
Summary information and primary citation
- PDB-id
- 5mga; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase
- Method
- X-ray (3.0 Å)
- Summary
- Structure of the cpf1 endonuclease r-loop complex after DNA cleavage
- Reference
- Stella S, Alcon P, Montoya G (2017): "Structure of the Cpf1 endonuclease R-loop complex after target DNA cleavage." Nature, 546, 559-563. doi: 10.1038/nature22398.
- Abstract
- Cpf1 is an RNA-guided endonuclease that is emerging as a powerful genome-editing tool. Here we provide insight into its DNA-targeting mechanism by determining the structure of Francisella novicida Cpf1 with the triple-stranded R-loop generated after DNA cleavage. The structure reveals the machinery involved in DNA unwinding to form a CRISPR RNA (crRNA)-DNA hybrid and a displaced DNA strand. The protospacer adjacent motif (PAM) is recognized by the PAM-interacting domain. The loop-lysine helix-loop motif in this domain contains three conserved lysine residues that are inserted in a dentate manner into the double-stranded DNA. Unzipping of the double-stranded DNA occurs in a cleft arranged by acidic and hydrophobic residues facilitating the crRNA-DNA hybrid formation. The PAM single-stranded DNA is funnelled towards the nuclease site through a mixed hydrophobic and basic cavity. In this catalytic conformation, the PAM-interacting domain and the helix-loop-helix motif in the REC1 domain adopt a 'rail' shape and 'flap-on' conformations, respectively, channelling the PAM strand into the cavity. A steric barrier between the RuvC-II and REC1 domains forms the 'septum', separating the displaced PAM strand and the crRNA-DNA hybrid, avoiding DNA re-annealing. Mutations in key residues reveal a mechanism linking the PAM and DNA nuclease sites. Analysis of the Cpf1 structures proposes a singular working model of RNA-guided DNA cleavage, suggesting new avenues for redesign of Cpf1.