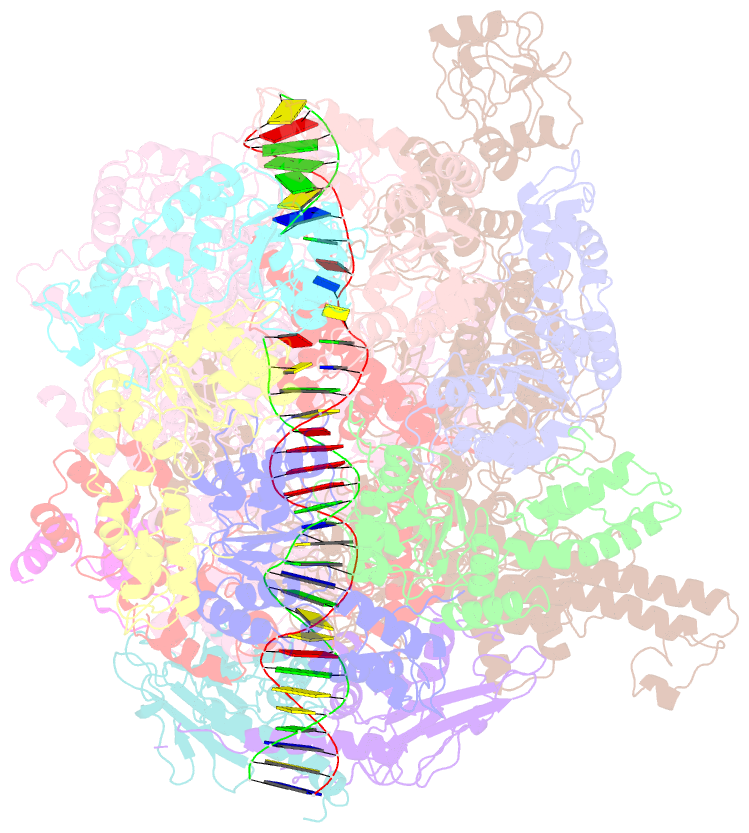
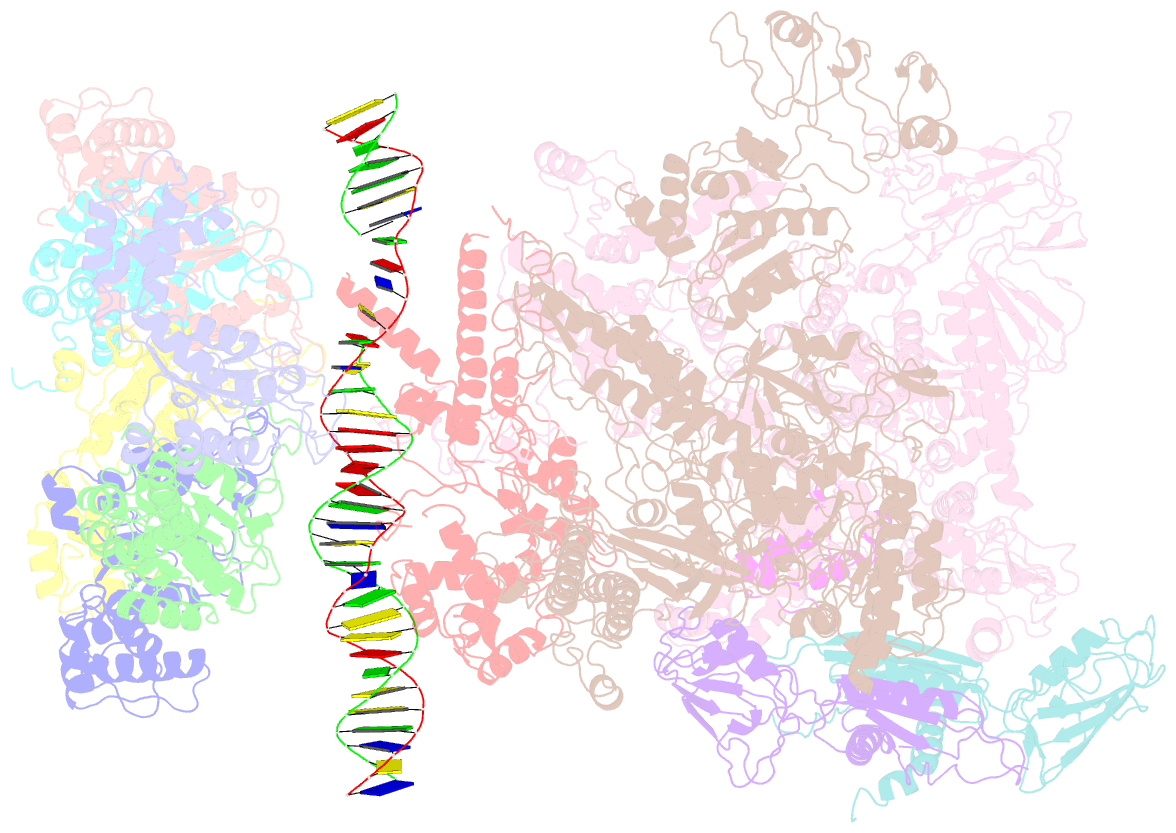
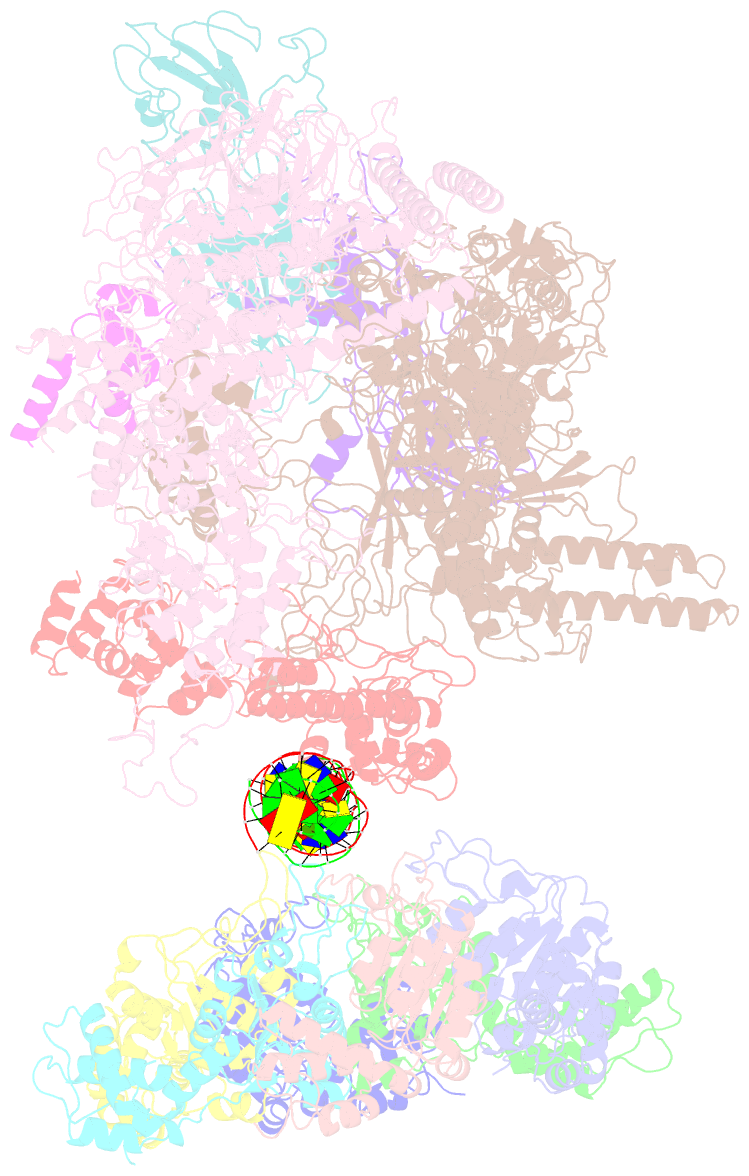
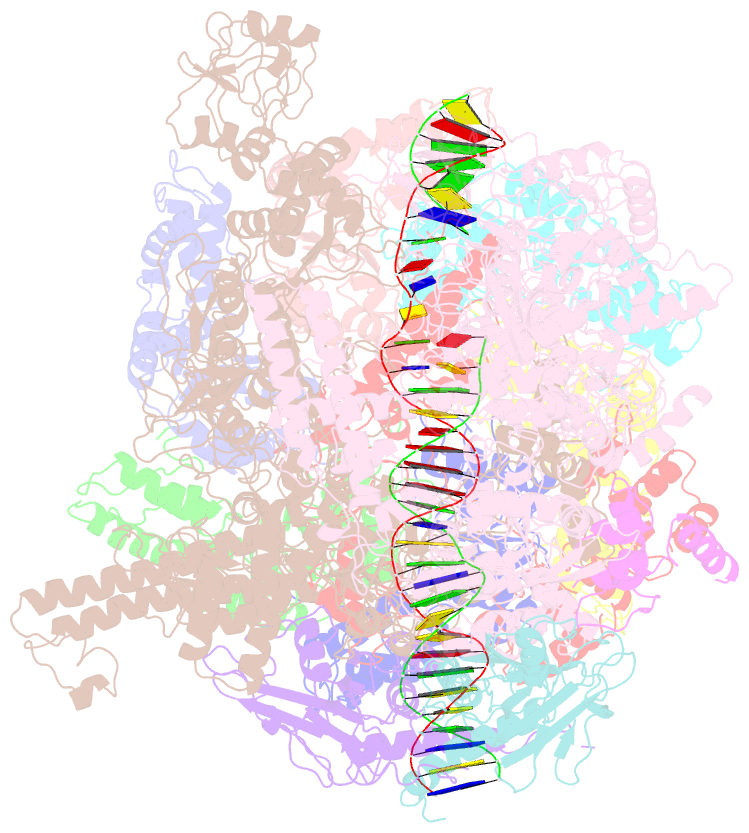
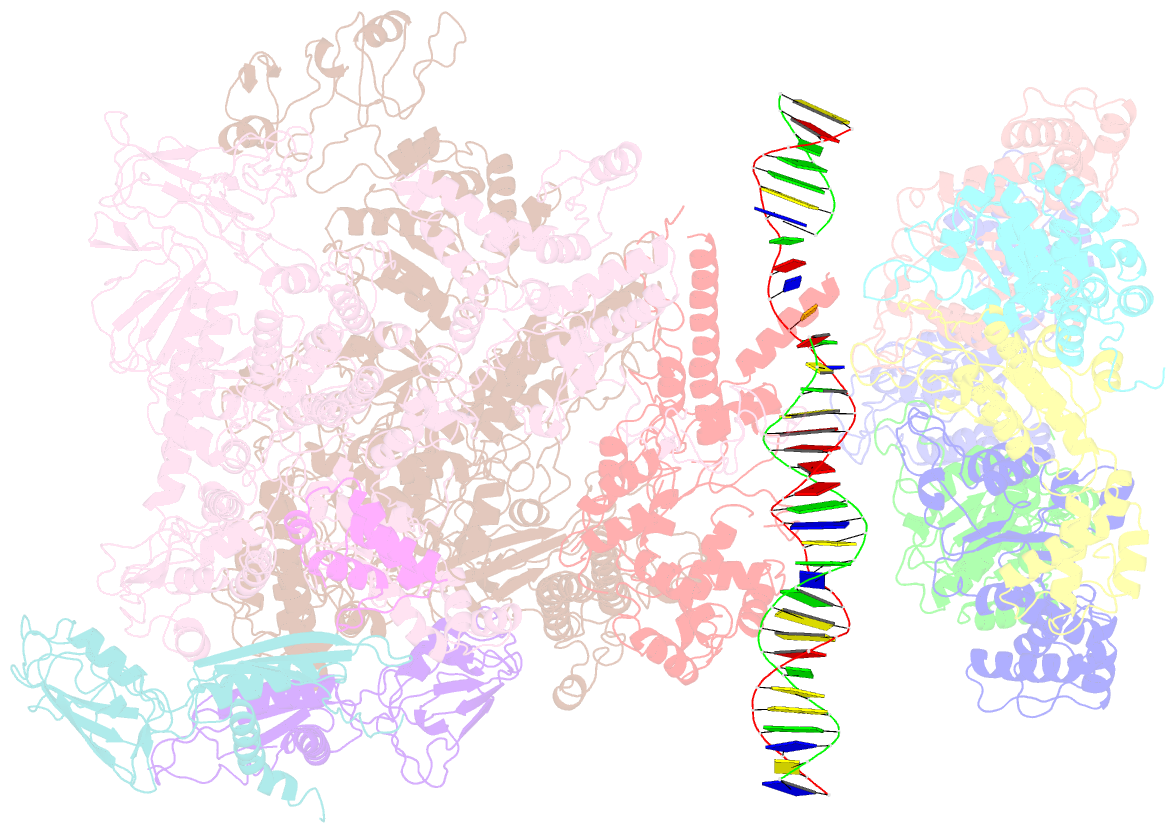
Summary information and primary citation
- PDB-id
- 5nss; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription
- Method
- cryo-EM (5.8 Å)
- Summary
- cryo-EM structure of RNA polymerase-sigma54 holoenzyme with promoter DNA and transcription activator pspf intermedate complex
- Reference
- Glyde R, Ye F, Darbari VC, Zhang N, Buck M, Zhang X (2017): "Structures of RNA Polymerase Closed and Intermediate Complexes Reveal Mechanisms of DNA Opening and Transcription Initiation." Mol. Cell, 67, 106-116.e4. doi: 10.1016/j.molcel.2017.05.010.
- Abstract
- Gene transcription is carried out by RNA polymerases (RNAPs). For transcription to occur, the closed promoter complex (RPc), where DNA is double stranded, must isomerize into an open promoter complex (RPo), where the DNA is melted out into a transcription bubble and the single-stranded template DNA is delivered to the RNAP active site. Using a bacterial RNAP containing the alternative σ54 factor and cryoelectron microscopy, we determined structures of RPc and the activator-bound intermediate complex en route to RPo at 3.8 and 5.8 Å. Our structures show how RNAP-σ54 interacts with promoter DNA to initiate the DNA distortions required for transcription bubble formation, and how the activator interacts with RPc, leading to significant conformational changes in RNAP and σ54 that promote RPo formation. We propose that DNA melting is an active process initiated in RPc and that the RNAP conformations of intermediates are significantly different from that of RPc and RPo.