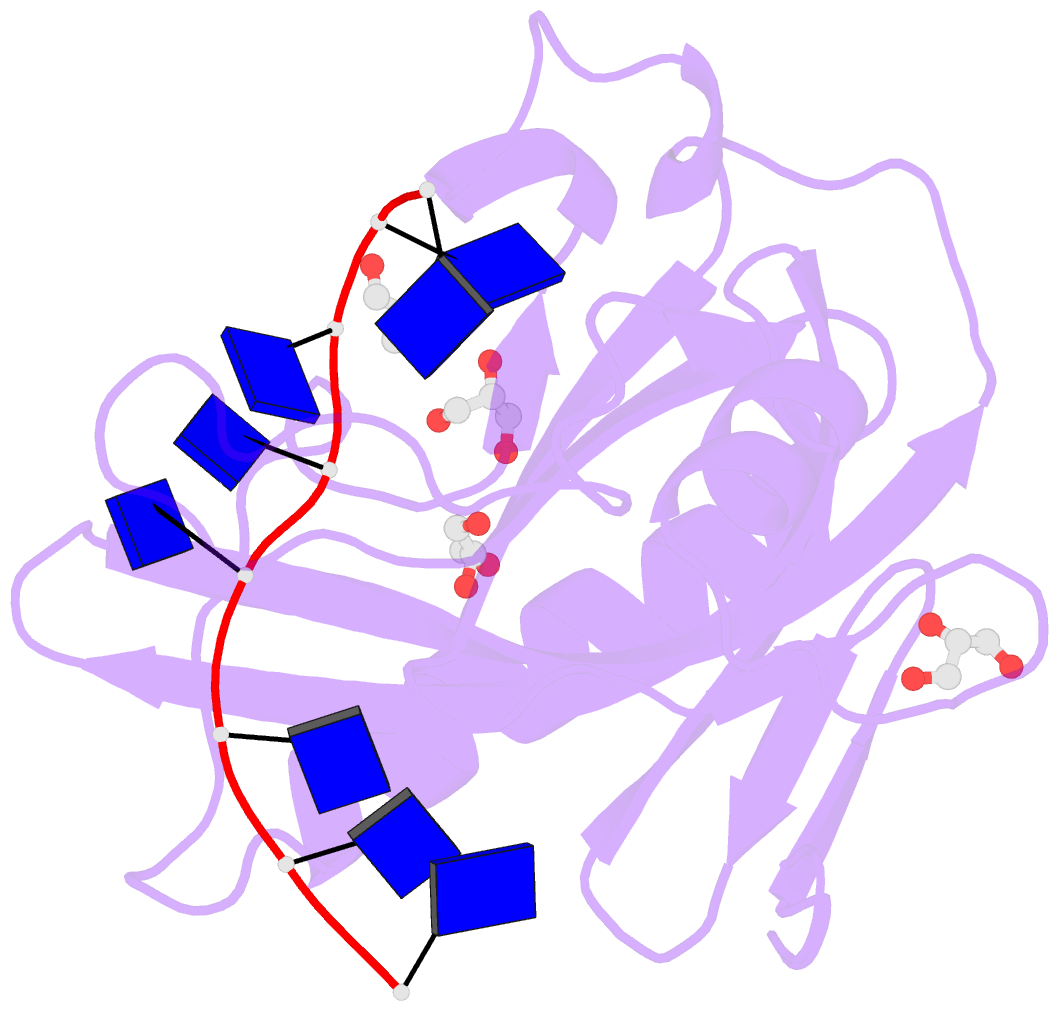
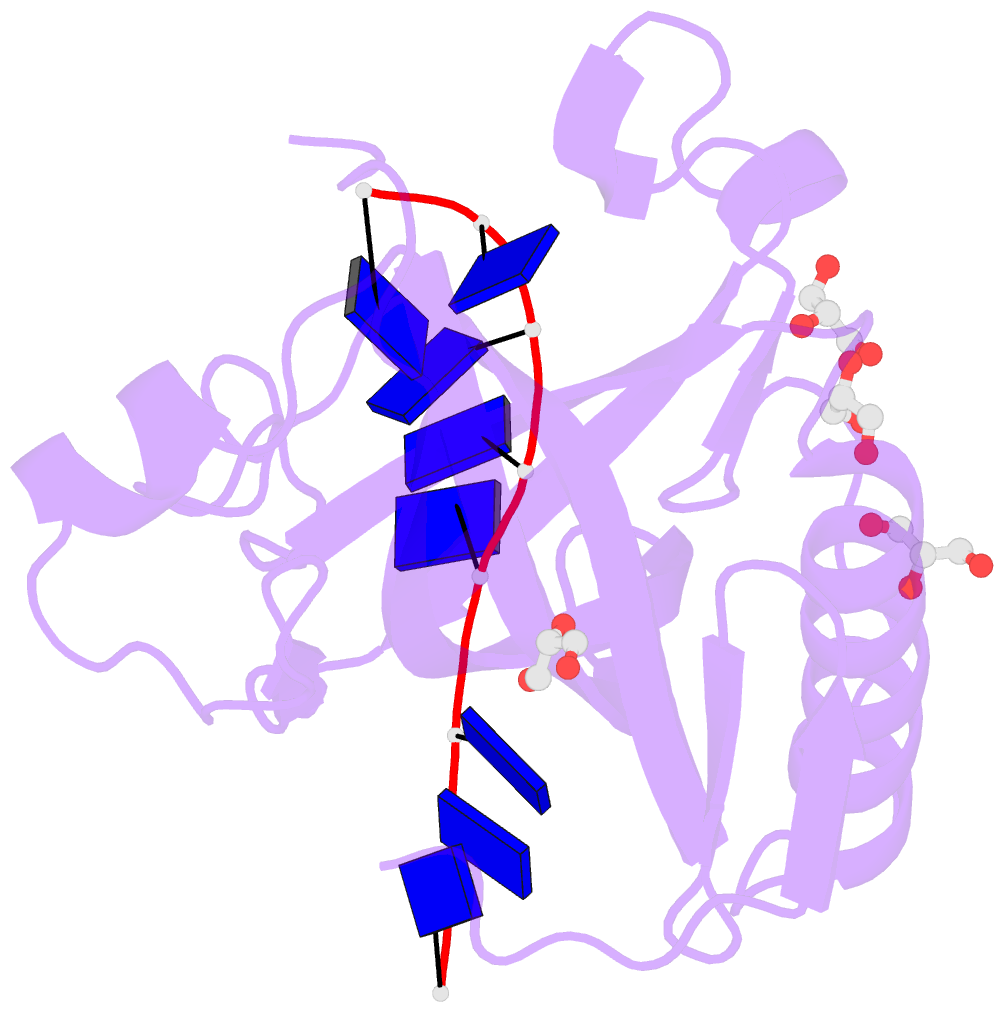
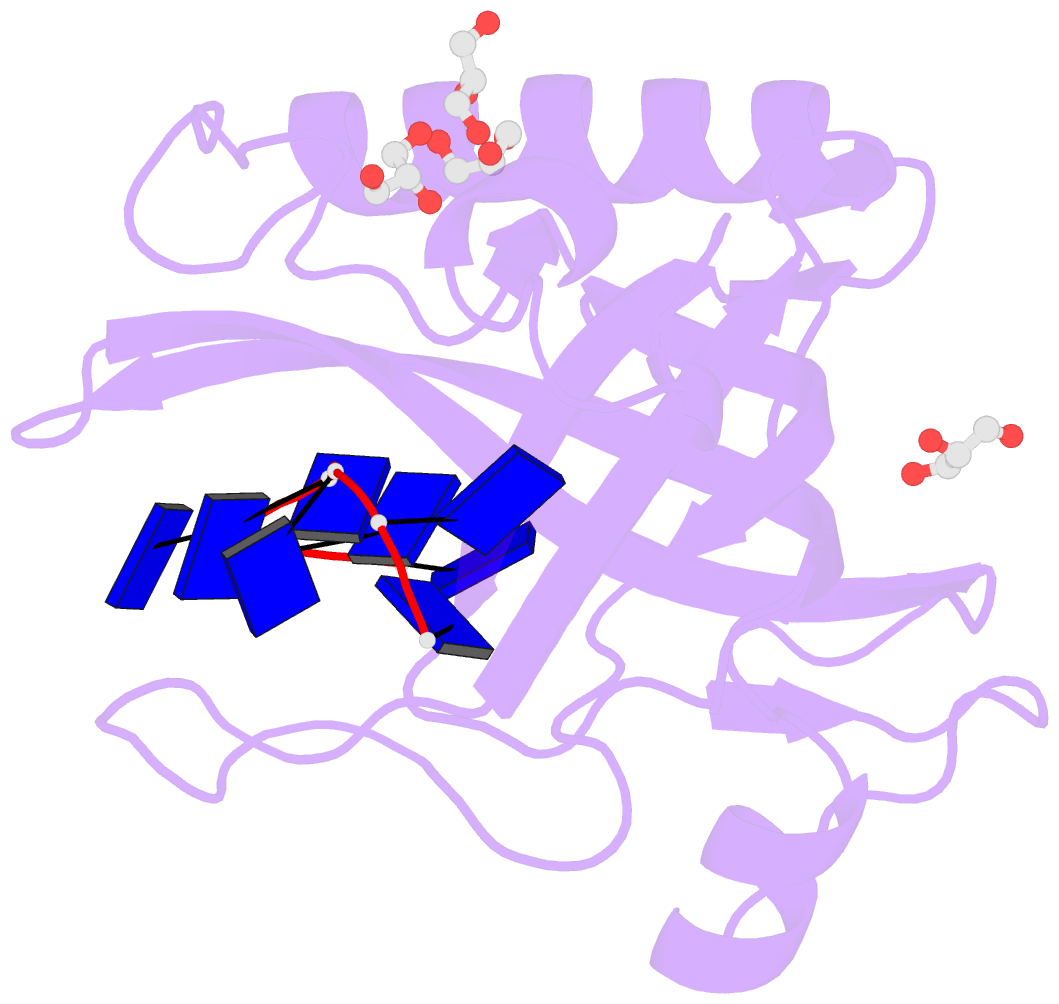
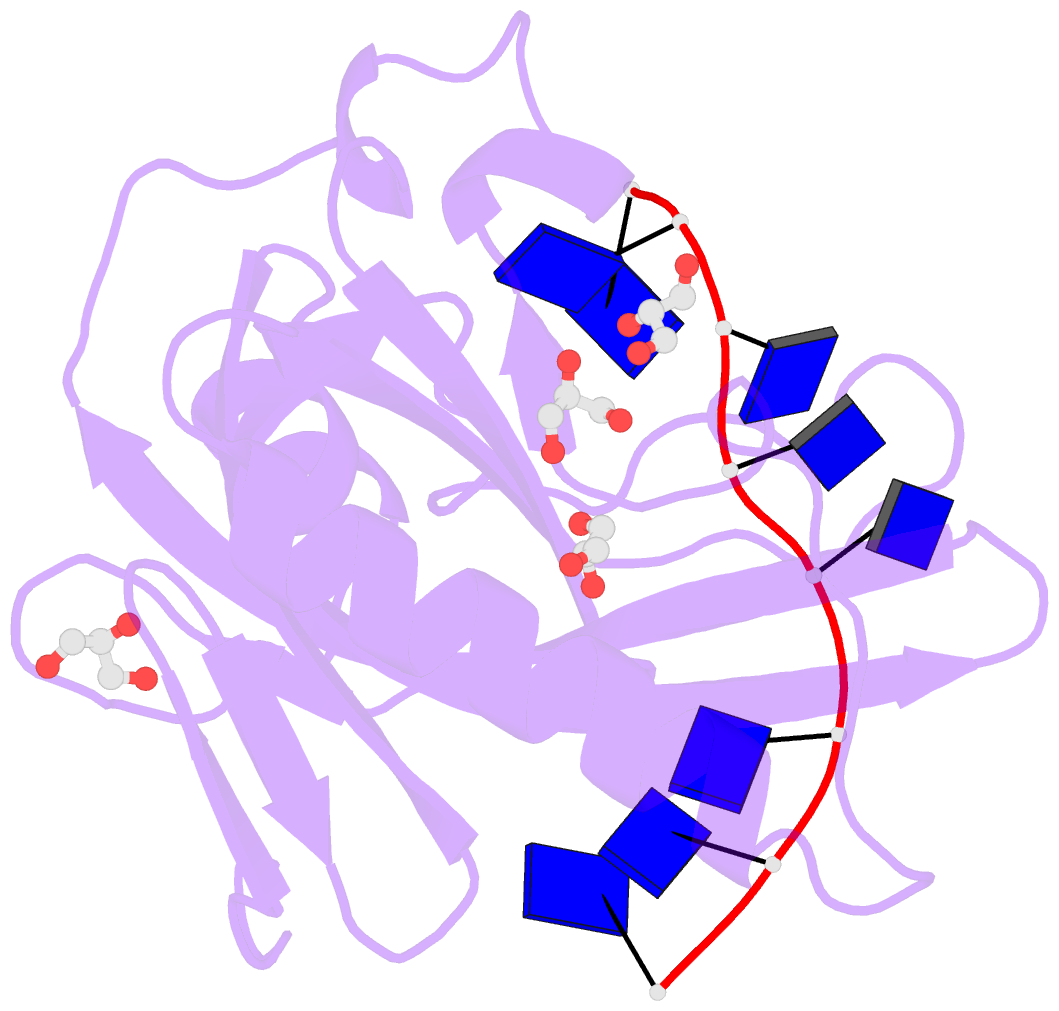
Summary information and primary citation
- PDB-id
- 5odl; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein
- Method
- X-ray (1.56 Å)
- Summary
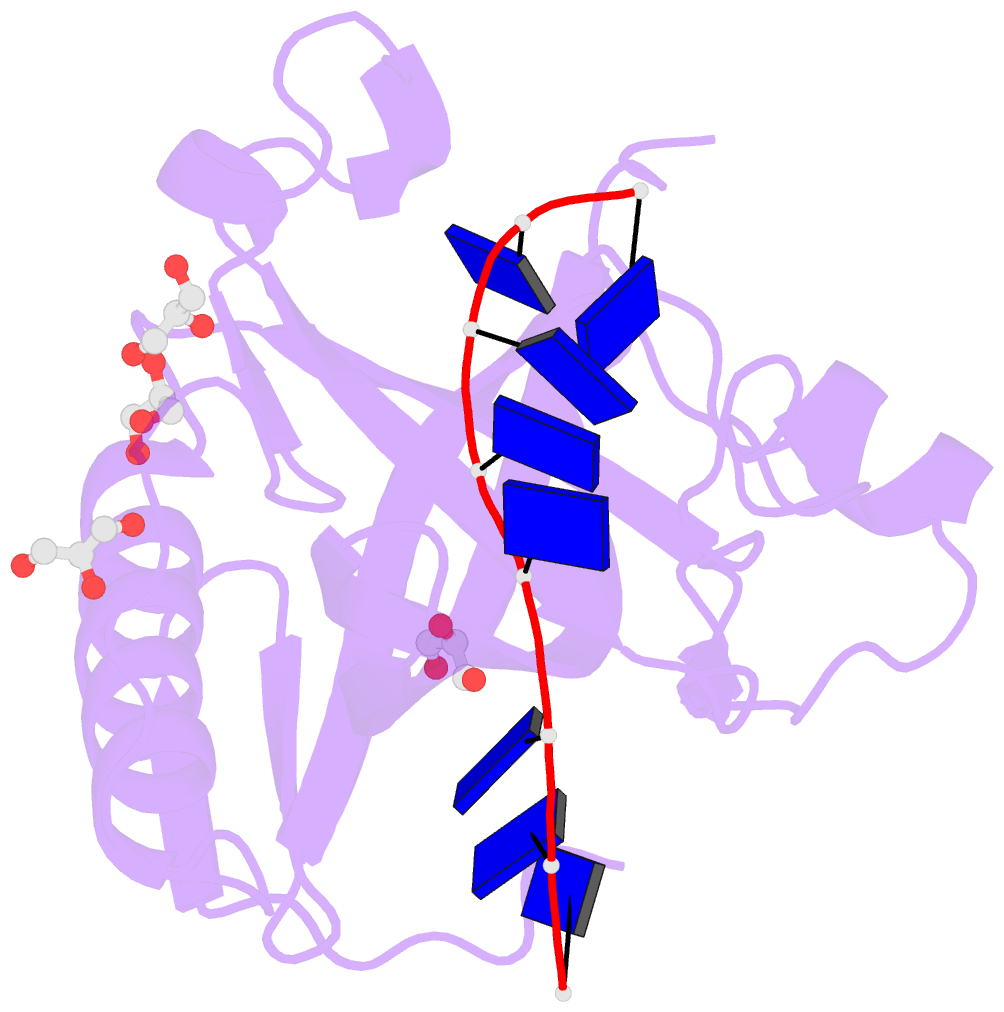
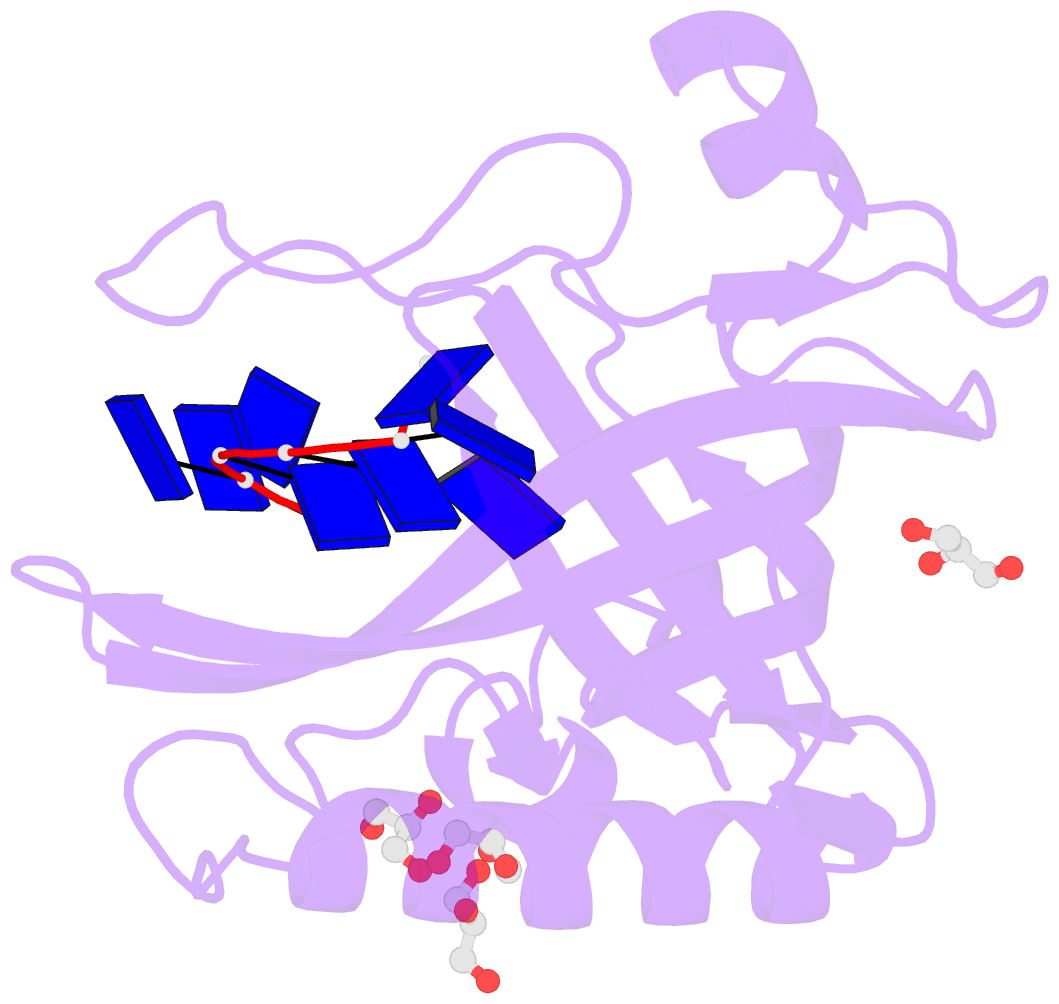
- Single-stranded DNA-binding protein from bacteriophage enc34 in complex with ssDNA
- Reference
- Cernooka E, Rumnieks J, Tars K, Kazaks A (2017): "Structural Basis for DNA Recognition of a Single-stranded DNA-binding Protein from Enterobacter Phage Enc34." Sci Rep, 7, 15529. doi: 10.1038/s41598-017-15774-y.
- Abstract
- Modern DNA sequencing capabilities have led to the discovery of a large number of new bacteriophage genomes, which are a rich source of novel proteins with an unidentified biological role. The genome of Enterobacter cancerogenus bacteriophage Enc34 contains several proteins of unknown function that are nevertheless conserved among distantly related phages. Here, we report the crystal structure of a conserved Enc34 replication protein ORF6 which contains a domain of unknown function DUF2815. Despite the low (~15%) sequence identity, the Enc34 ORF6 structurally resembles the gene 2.5 protein from bacteriophage T7, and likewise is a single-stranded DNA (ssDNA)-binding protein (SSB) that consists of a variation of the oligosaccharide/oligonucleotide-binding (OB)-fold and an unstructured C-terminal segment. We further report the crystal structure of a C-terminally truncated ORF6 in complex with an ssDNA oligonucleotide that reveals a DNA-binding mode involving two aromatic stacks and multiple electrostatic interactions, with implications for a common ssDNA recognition mechanism for all T7-type SSBs.