Summary information and primary citation
- PDB-id
- 5t14; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase-DNA
- Method
- X-ray (3.0 Å)
- Summary
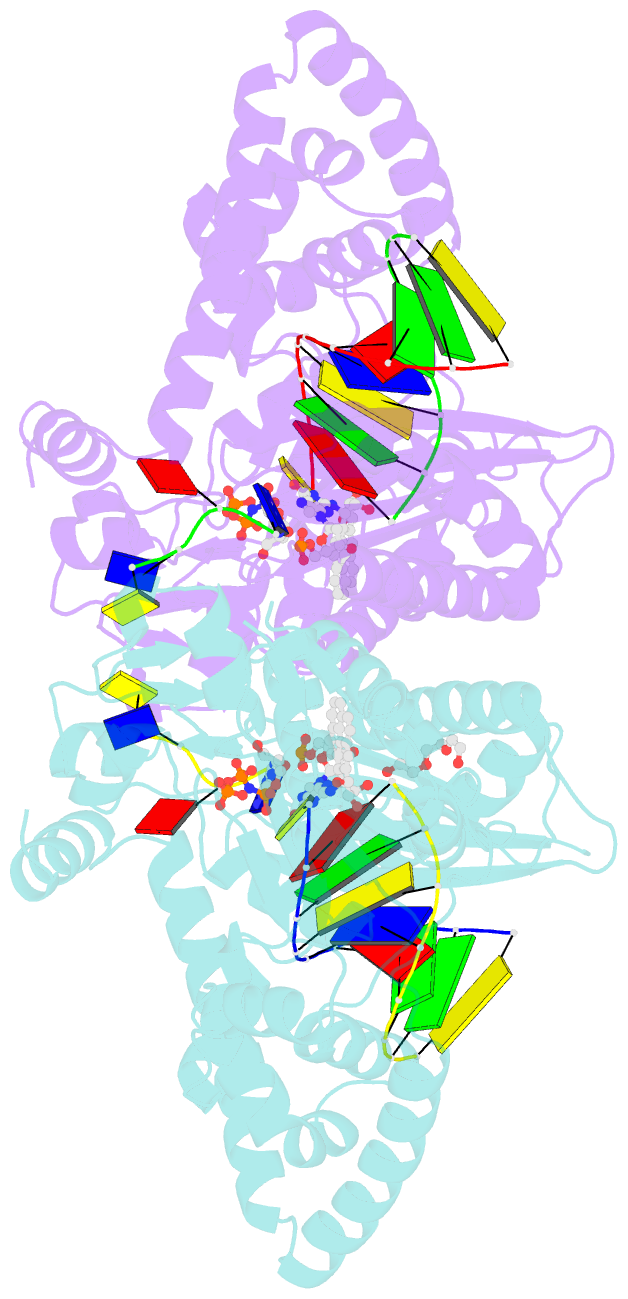
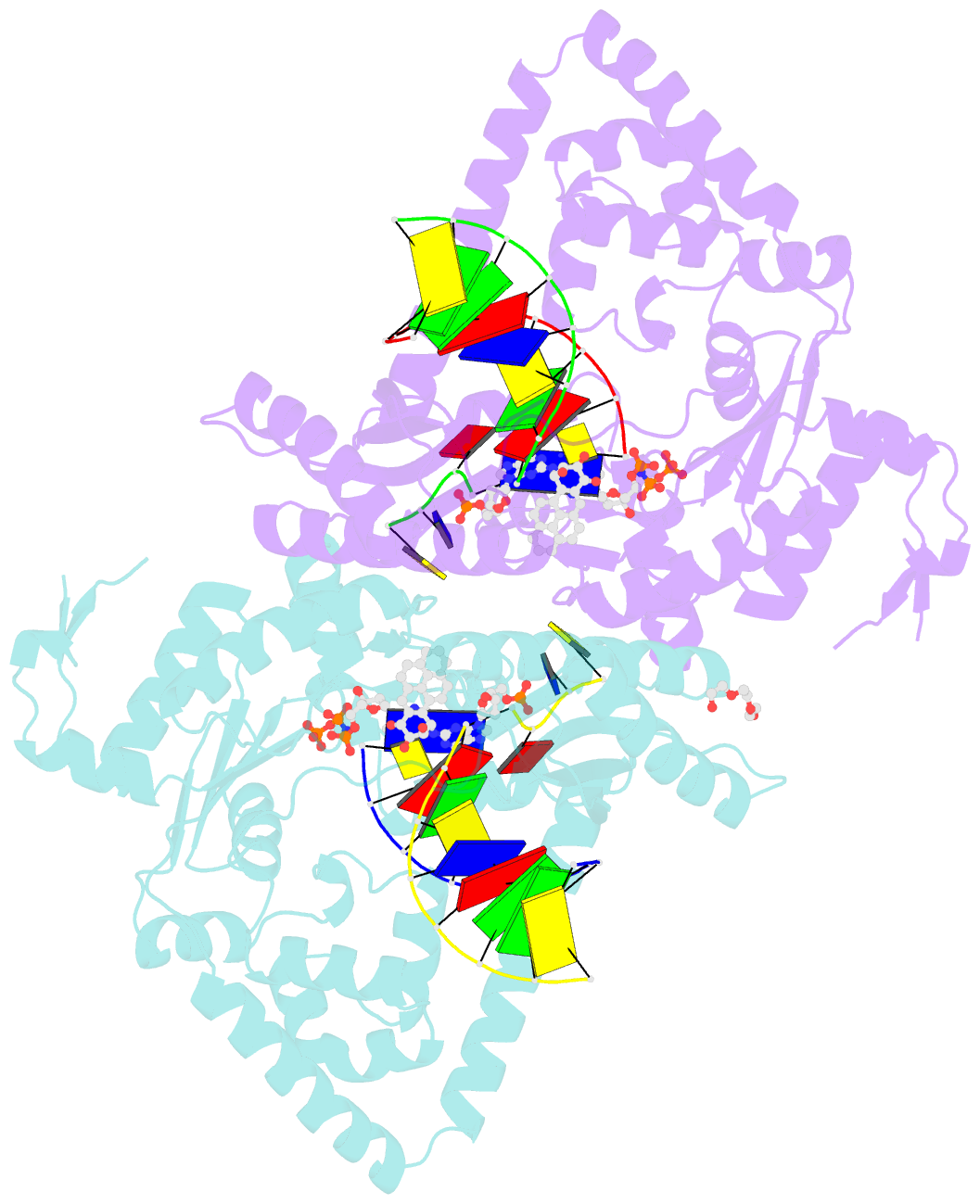
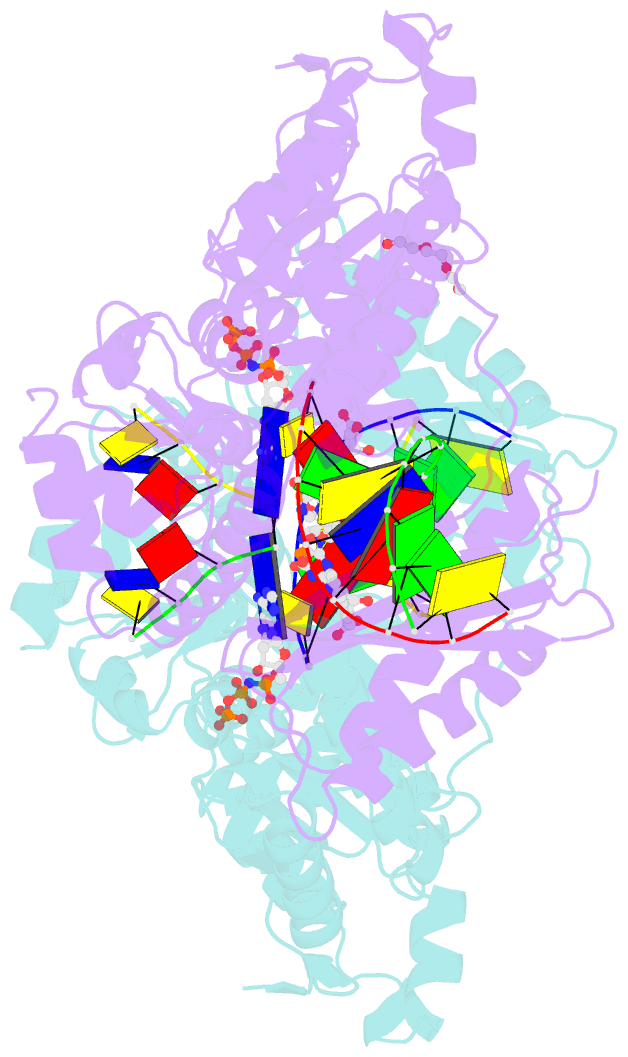
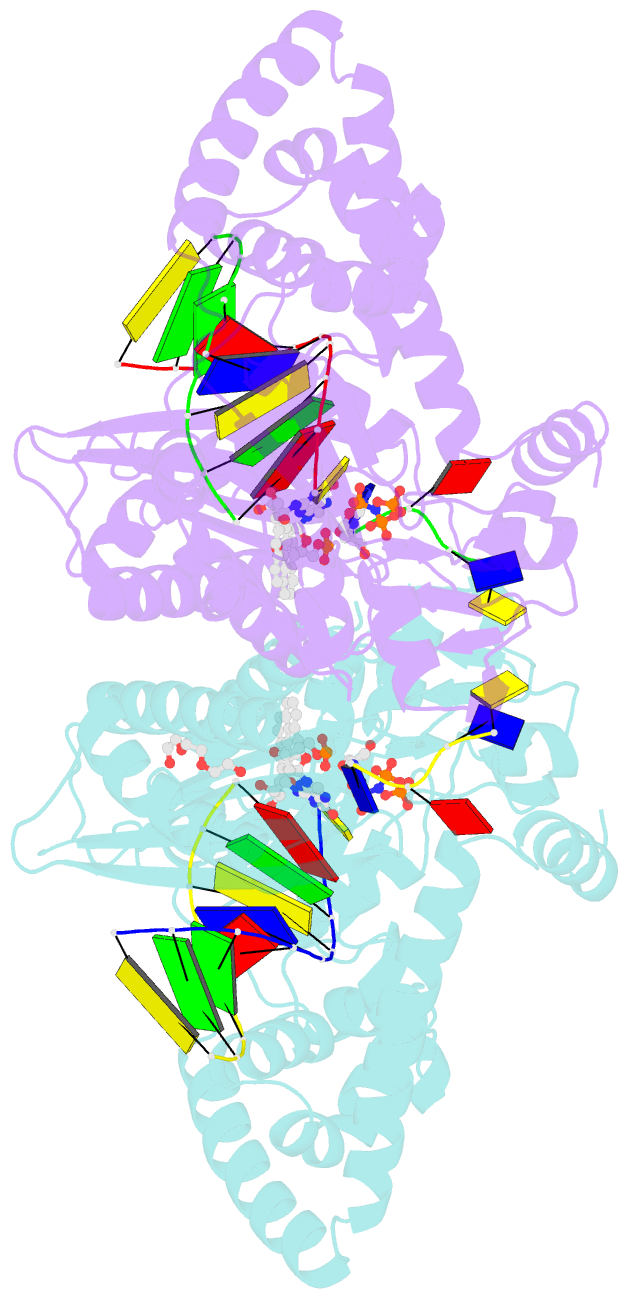
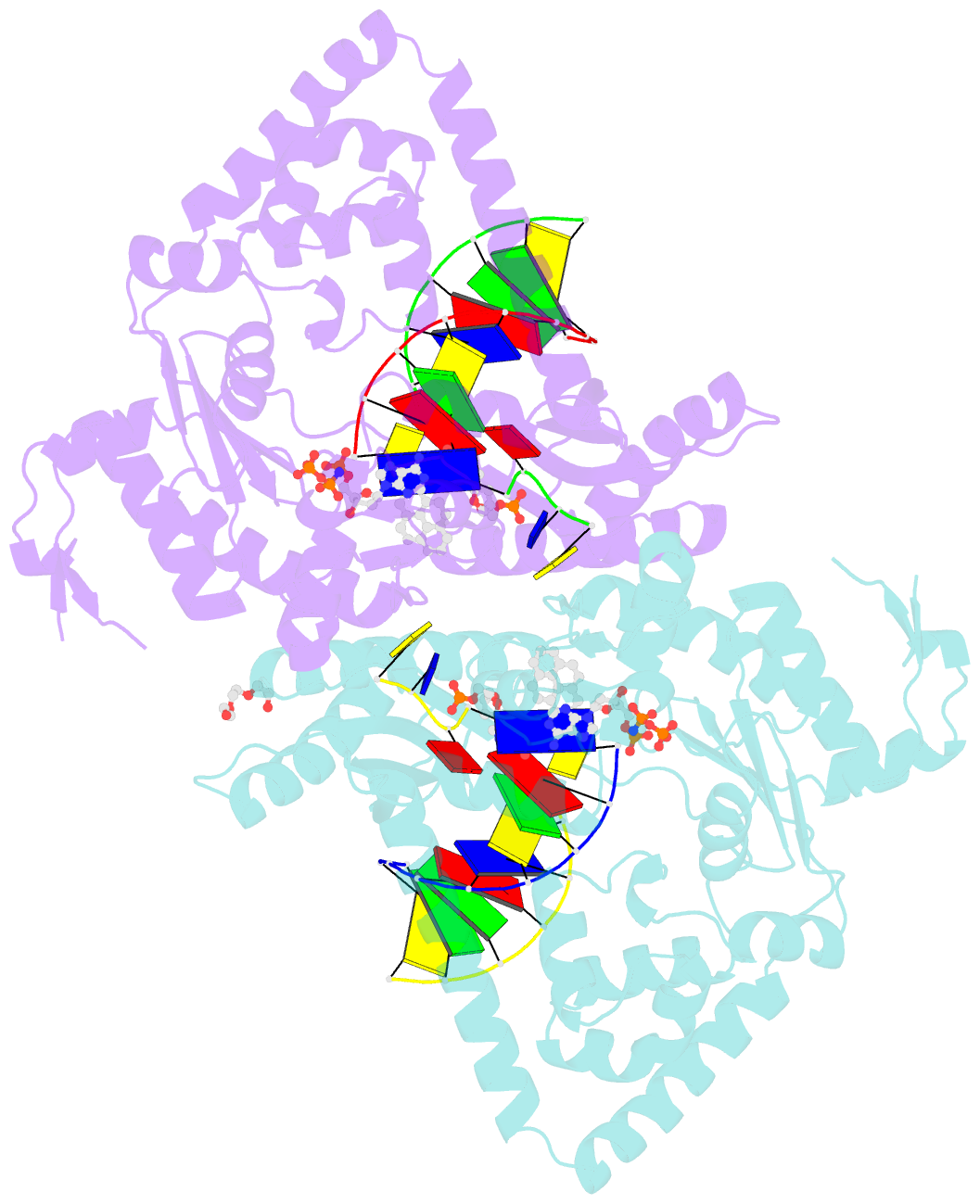
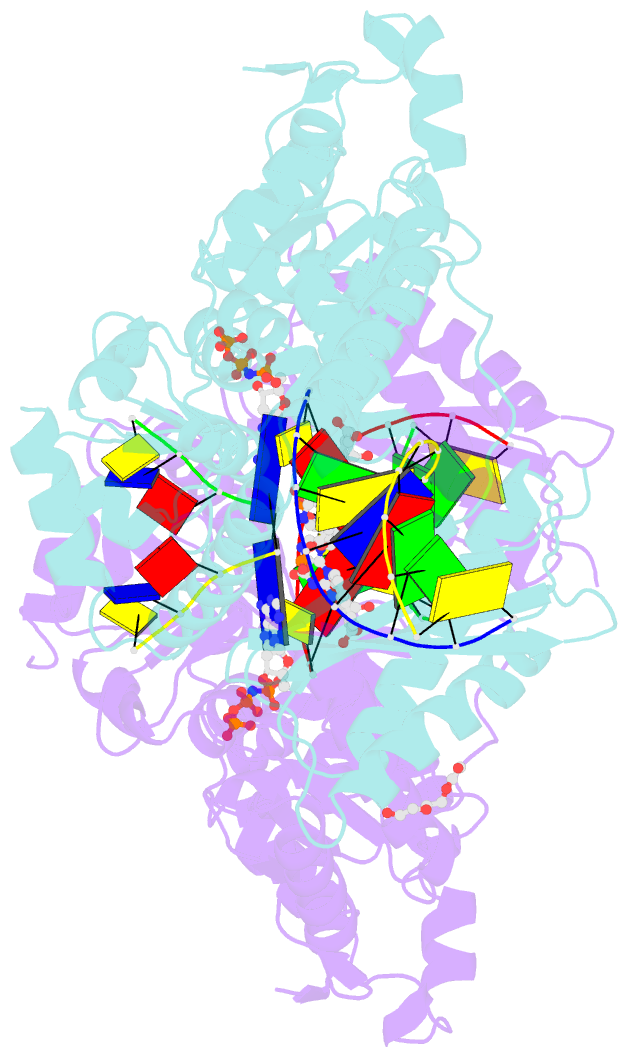
- DNA polymerase kappa extending beyond a bulky major benzo[a]pyrene adduct
- Reference
- Jha V, Ling H (2017): "Structural basis of accurate replication beyond a bulky major benzo[a]pyrene adduct by human DNA polymerase kappa." DNA Repair (Amst.), 49, 43-50. doi: 10.1016/j.dnarep.2016.11.001.
- Abstract
- Human Y-family DNA polymerase kappa (polκ) is specialized to bypass bulky lesions in DNA in an error-free way, thus protecting cells from carcinogenic bulky DNA adducts. Benzo[a]pyrene (BP) is one of the most ubiquitous polycyclic aromatic hydrocarbons and an environmental carcinogen. BP covalently modifies DNA and generates mutagenic, bulky adducts. The major BP adduct formed in cells is 10S (+)-trans-anti-BP-N2-dG adduct (BP-dG), which is associated with cancer. The molecular mechanism of how polκ replicates BP-dG accurately is not clear. Here we report the structure of polκ captured at the lesion-extension stage: the enzyme is extending the primer strand after the base pair containing the BP-dG adduct in the template strand at the -1 position. Polκ accommodates the BP adduct in the nascent DNA's minor groove and keeps the adducted DNA helix in a B-form. Two water molecules cover the edge of the minor groove of the replicating base pair (0 position), which is secured by the BP ring in the -1 position in a 5' orientation. The 5' oriented BP adduct keeps correct Watson-Crick base pairing in the active site and promotes high fidelity replication. Our structural and biochemical data reveal a unique molecular basis for accurate DNA replication right after the bulky lesion BP-dG.