Summary information and primary citation
- PDB-id
- 5t5c; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (1.851 Å)
- Summary
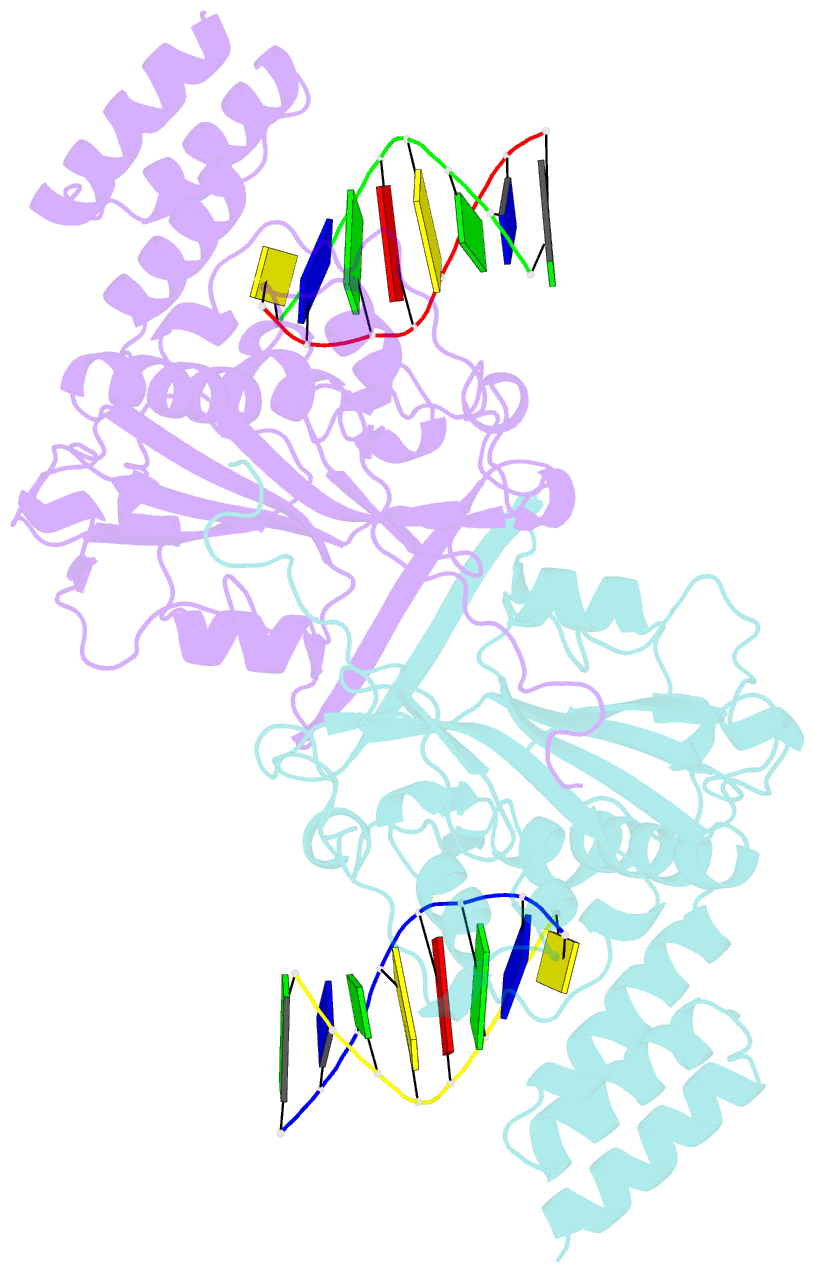
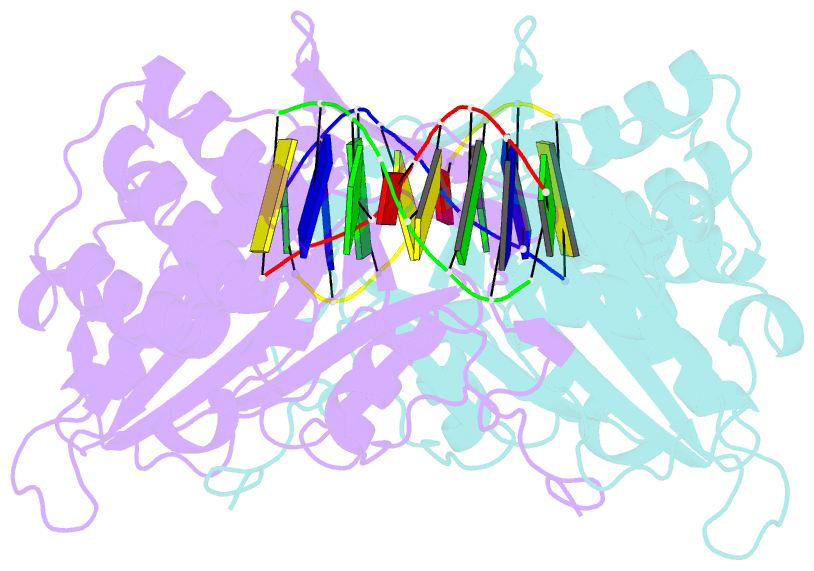
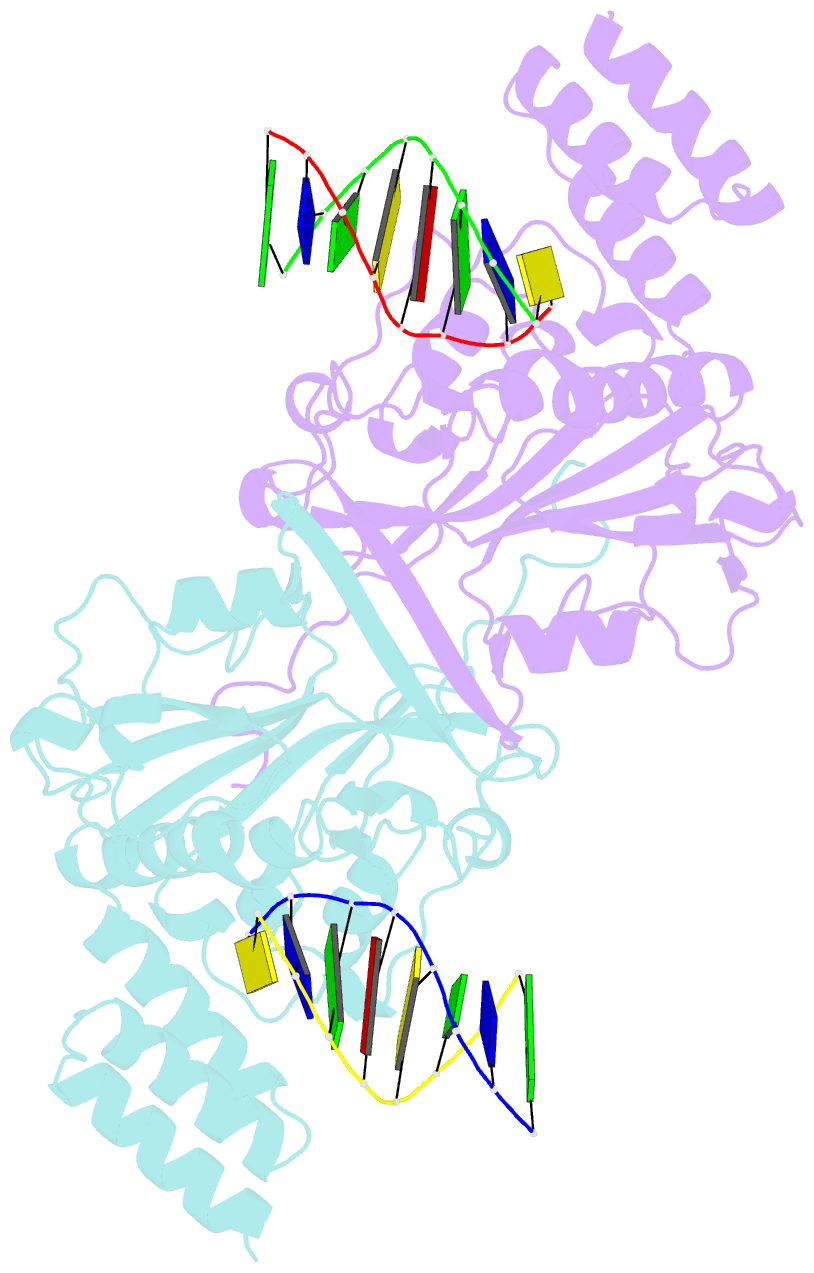
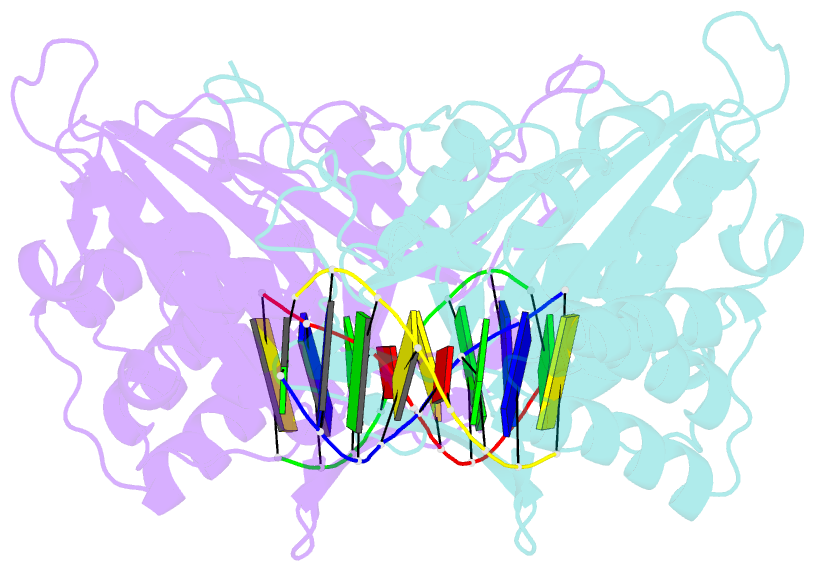
- A novel domain in human exog converts apoptotic endonuclease to DNA-repair enzyme
- Reference
- Szymanski MR, Yu W, Gmyrek AM, White MA, Molineux IJ, Lee JC, Yin YW (2017): "A domain in human EXOG converts apoptotic endonuclease to DNA-repair exonuclease." Nat Commun, 8, 14959. doi: 10.1038/ncomms14959.
- Abstract
- Human EXOG (hEXOG) is a 5'-exonuclease that is crucial for mitochondrial DNA repair; the enzyme belongs to a nonspecific nuclease family that includes the apoptotic endonuclease EndoG. Here we report biochemical and structural studies of hEXOG, including structures in its apo form and in a complex with DNA at 1.81 and 1.85 Å resolution, respectively. A Wing domain, absent in other ββα-Me members, suppresses endonuclease activity, but confers on hEXOG a strong 5'-dsDNA exonuclease activity that precisely excises a dinucleotide using an intrinsic 'tape-measure'. The symmetrical apo hEXOG homodimer becomes asymmetrical upon binding to DNA, providing a structural basis for how substrate DNA bound to one active site allosterically regulates the activity of the other. These properties of hEXOG suggest a pathway for mitochondrial BER that provides an optimal substrate for subsequent gap-filling synthesis by DNA polymerase γ.