Summary information and primary citation
- PDB-id
- 5tyc; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase-DNA
- Method
- X-ray (2.101 Å)
- Summary
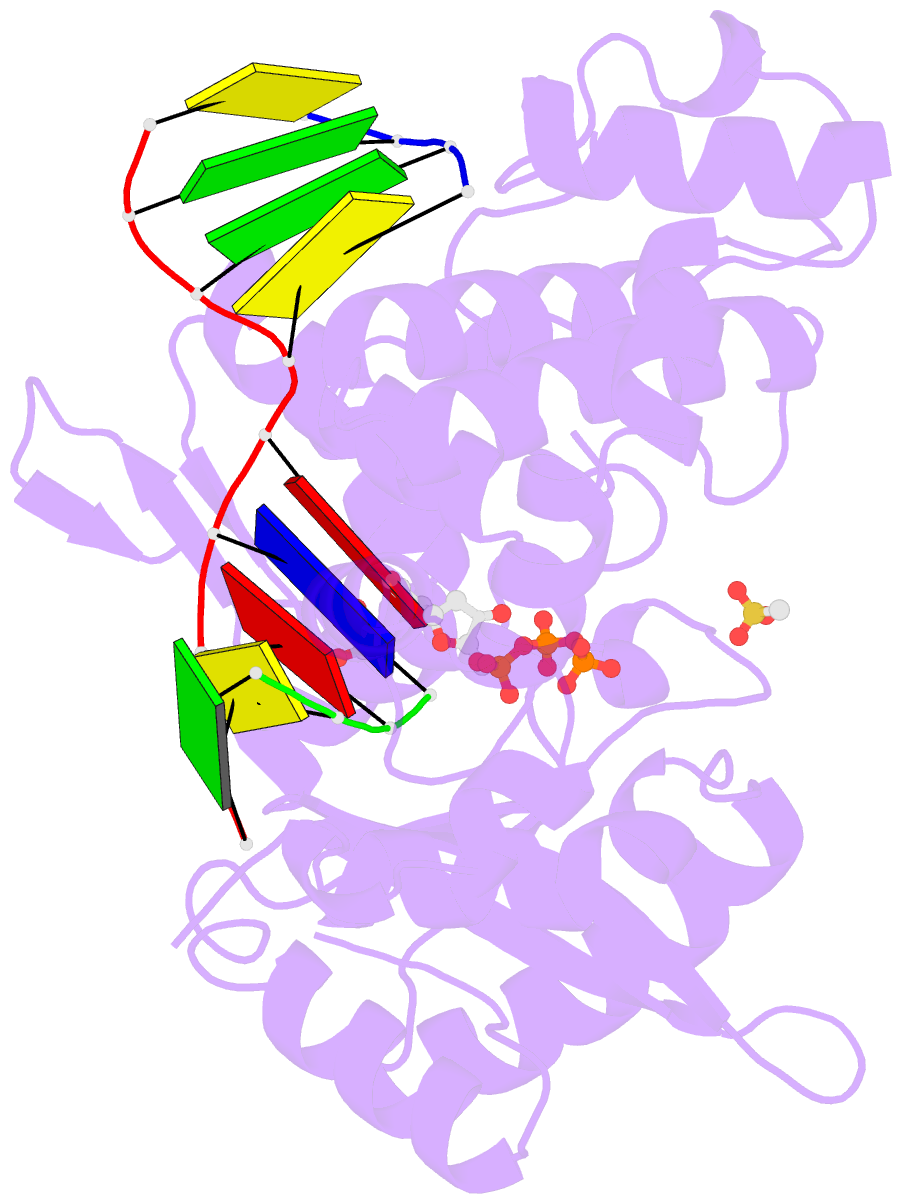
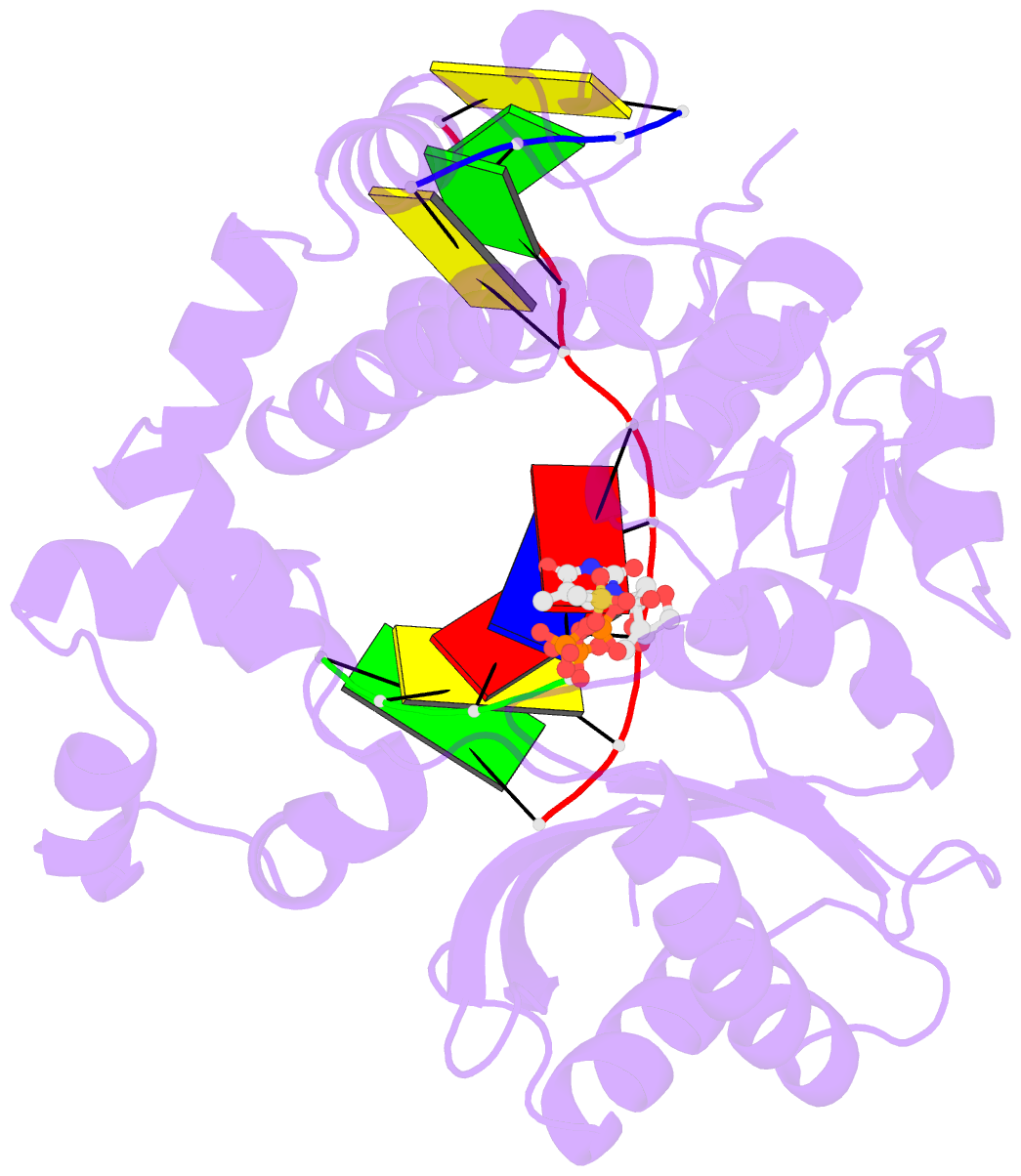
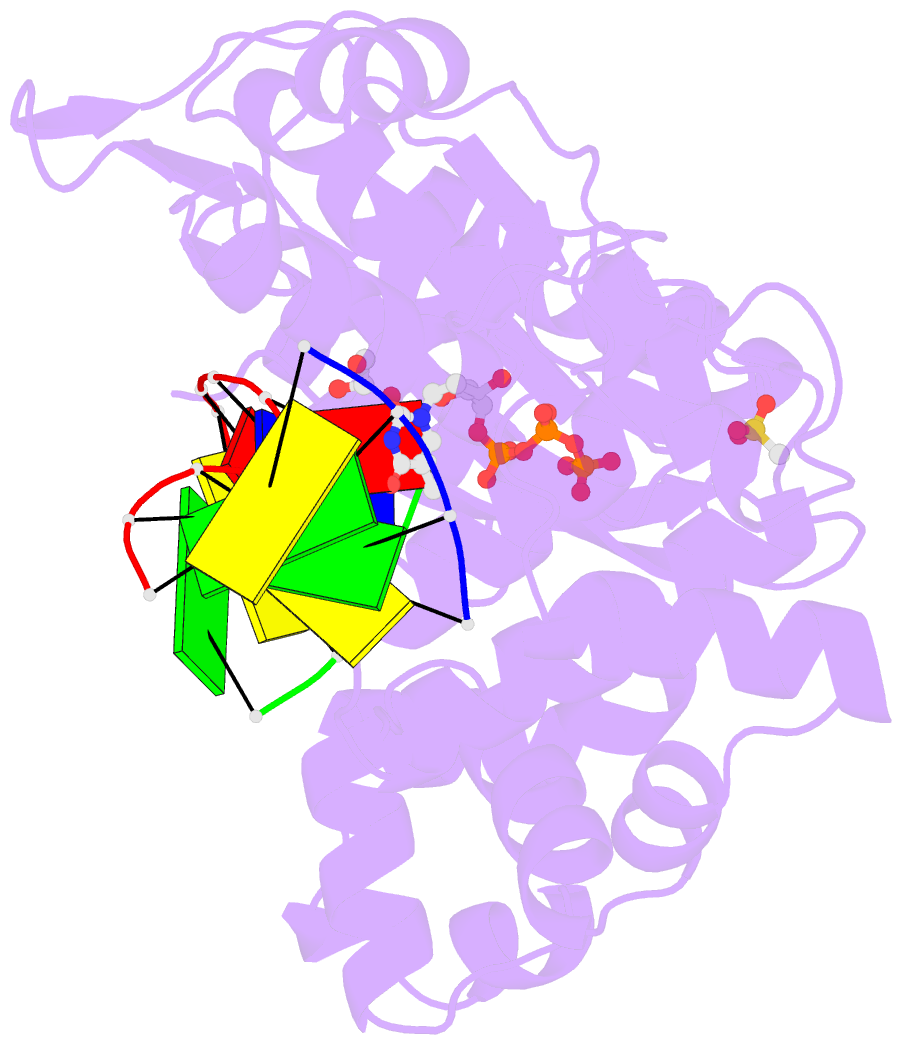
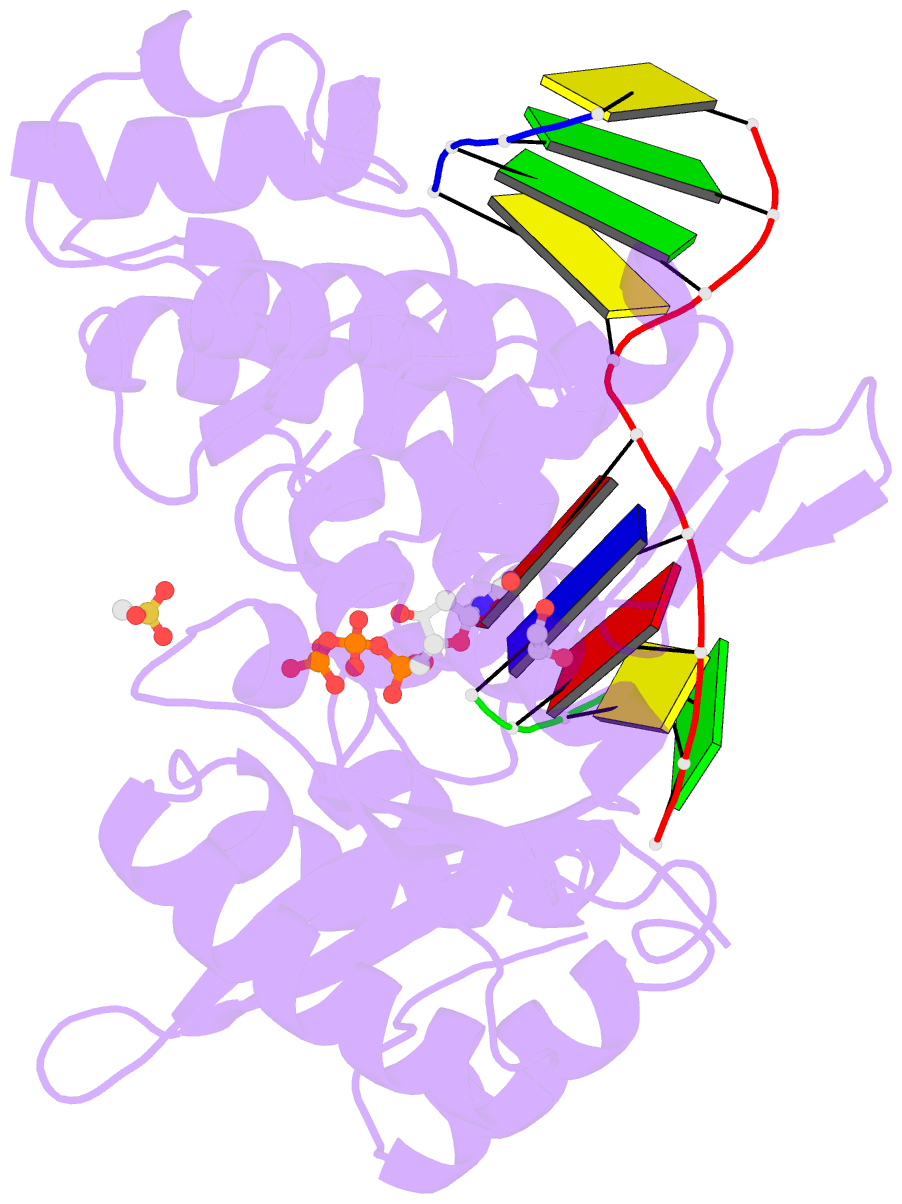
- DNA polymerase mu reactant complex, 10mm mg2+ (15 min)
- Reference
- Jamsen JA, Beard WA, Pedersen LC, Shock DD, Moon AF, Krahn JM, Bebenek K, Kunkel TA, Wilson SH (2017): "Time-lapse crystallography snapshots of a double-strand break repair polymerase in action." Nat Commun, 8, 253. doi: 10.1038/s41467-017-00271-7.
- Abstract
- DNA polymerase (pol) μ is a DNA-dependent polymerase that incorporates nucleotides during gap-filling synthesis in the non-homologous end-joining pathway of double-strand break repair. Here we report time-lapse X-ray crystallography snapshots of catalytic events during gap-filling DNA synthesis by pol μ. Unique catalytic intermediates and active site conformational changes that underlie catalysis are uncovered, and a transient third (product) metal ion is observed in the product state. The product manganese coordinates phosphate oxygens of the inserted nucleotide and PPi. The product metal is not observed during DNA synthesis in the presence of magnesium. Kinetic analyses indicate that manganese increases the rate constant for deoxynucleoside 5'-triphosphate insertion compared to magnesium. The likely product stabilization role of the manganese product metal in pol μ is discussed. These observations provide insight on structural attributes of this X-family double-strand break repair polymerase that impact its biological function in genome maintenance.DNA polymerase (pol) μ functions in DNA double-strand break repair. Here the authors use time-lapse X-ray crystallography to capture the states of pol µ during the conversion from pre-catalytic to product complex and observe a third transiently bound metal ion in the product state.