Summary information and primary citation
- PDB-id
- 5udz; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein-RNA
- Method
- X-ray (2.0 Å)
- Summary
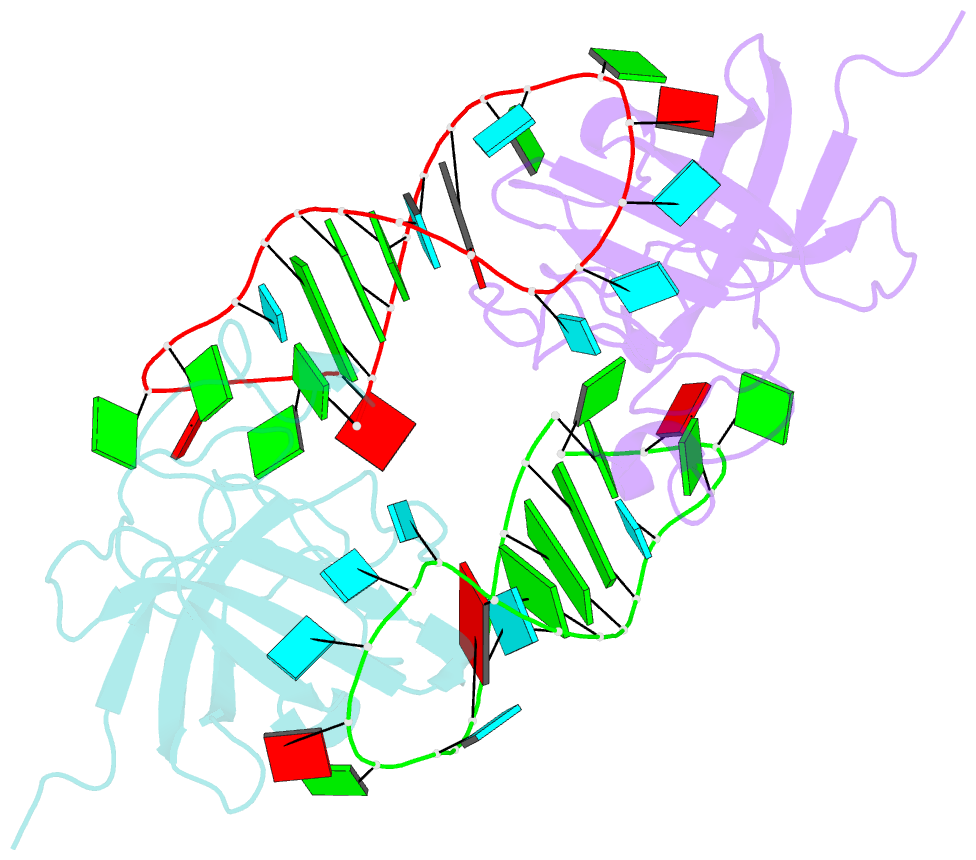
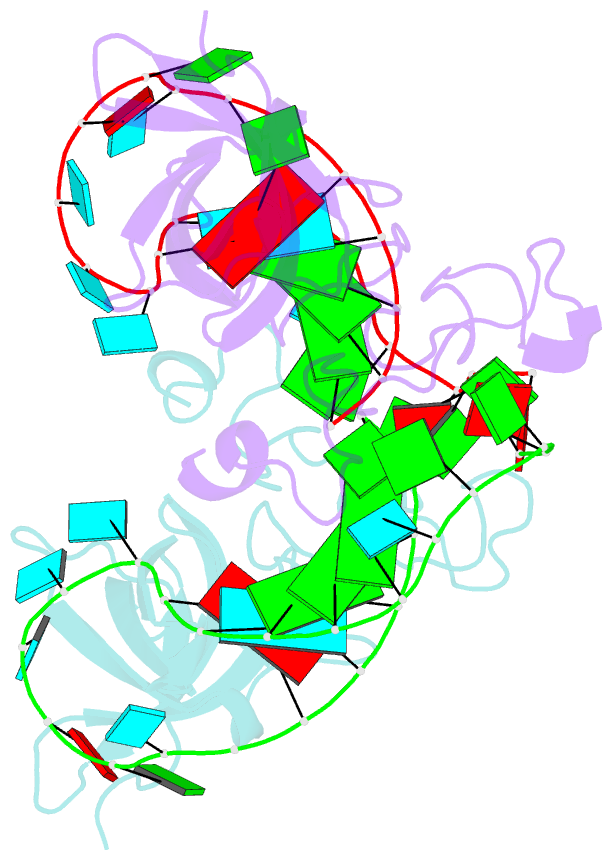
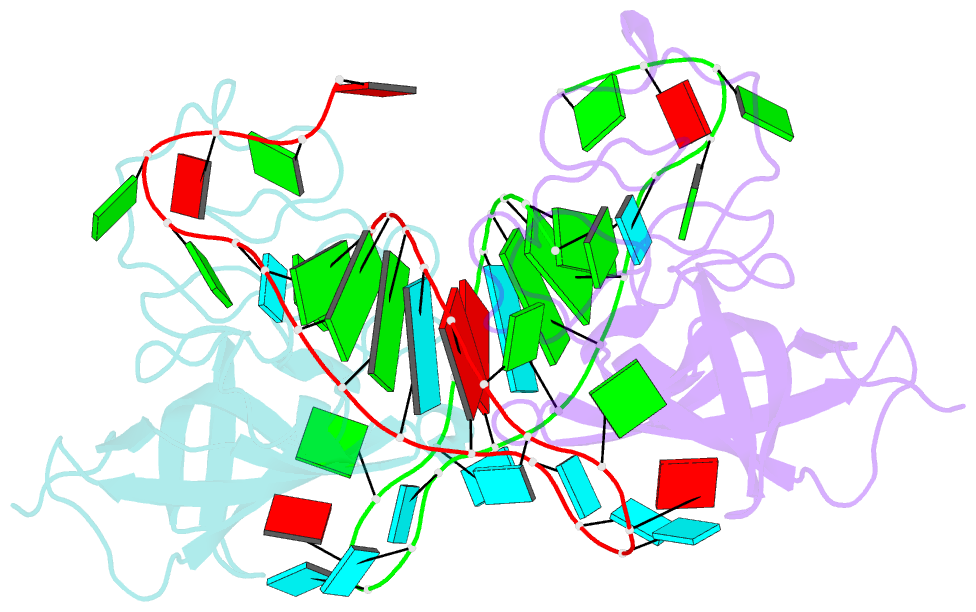
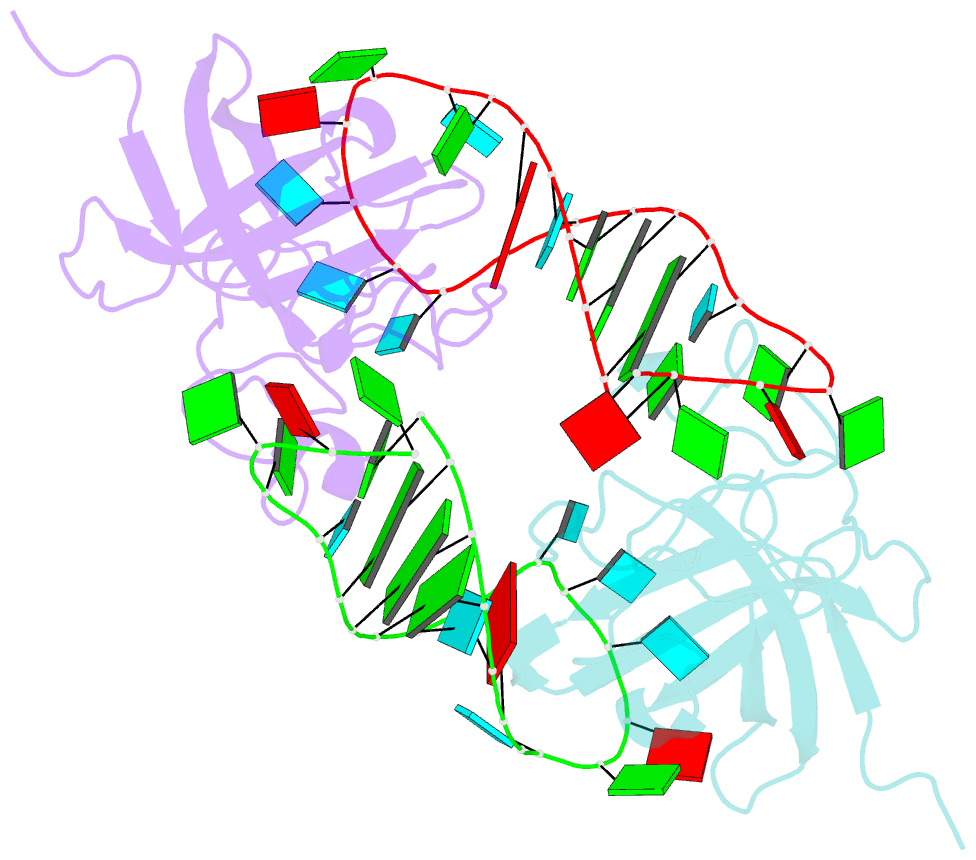
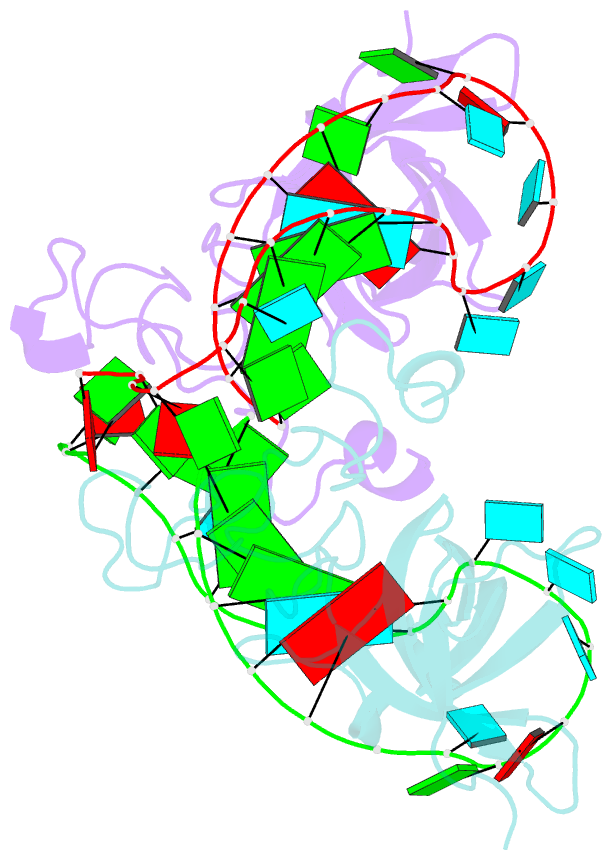
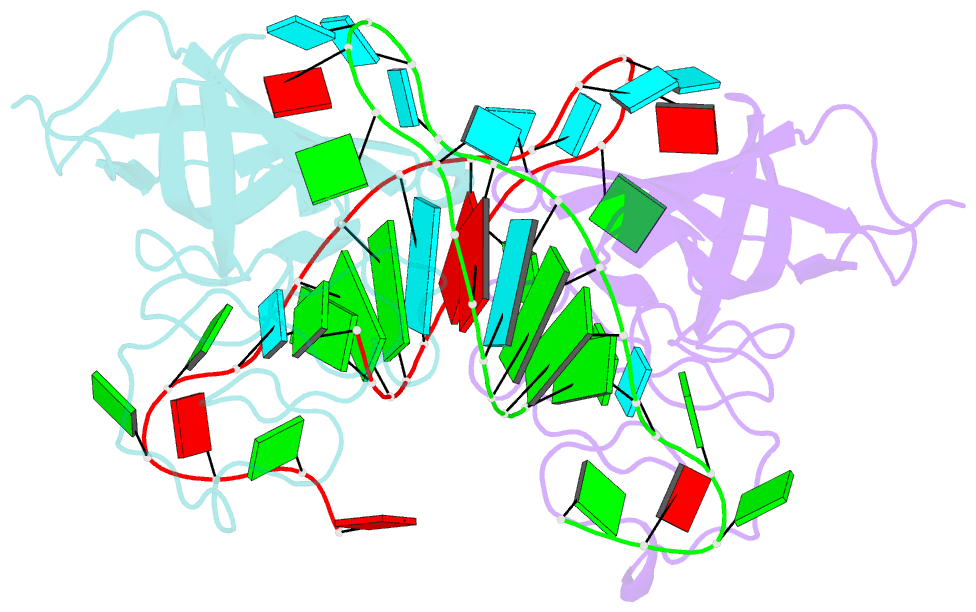
- Human lin28a in complex with let-7f-1 microrna pre-element
- Reference
- Wang L, Nam Y, Lee AK, Yu C, Roth K, Chen C, Ransey EM, Sliz P (2017): "LIN28 Zinc Knuckle Domain Is Required and Sufficient to Induce let-7 Oligouridylation." Cell Rep, 18, 2664-2675. doi: 10.1016/j.celrep.2017.02.044.
- Abstract
- LIN28 is an RNA binding protein that plays crucial roles in pluripotency, glucose metabolism, tissue regeneration, and tumorigenesis. LIN28 binds to the let-7 primary and precursor microRNAs through bipartite recognition and induces degradation of let-7 precursors (pre-let-7) by promoting oligouridylation by terminal uridylyltransferases (TUTases). Here, we report that the zinc knuckle domain (ZKD) of mouse LIN28 recruits TUT4 to initiate the oligouridylation of let-7 precursors. Our crystal structure of human LIN28 in complex with a fragment of pre-let-7f-1 determined to 2.0 Å resolution shows that the interaction between ZKD and RNA is constrained to a small cavity with a high druggability score. We demonstrate that the specific interaction between ZKD and pre-let-7 is necessary and sufficient to induce oligouridylation by recruiting the N-terminal fragment of TUT4 (NTUT4) and the formation of a stable ZKD:NTUT4:pre-let-7 ternary complex is crucial for the acquired processivity of TUT4.