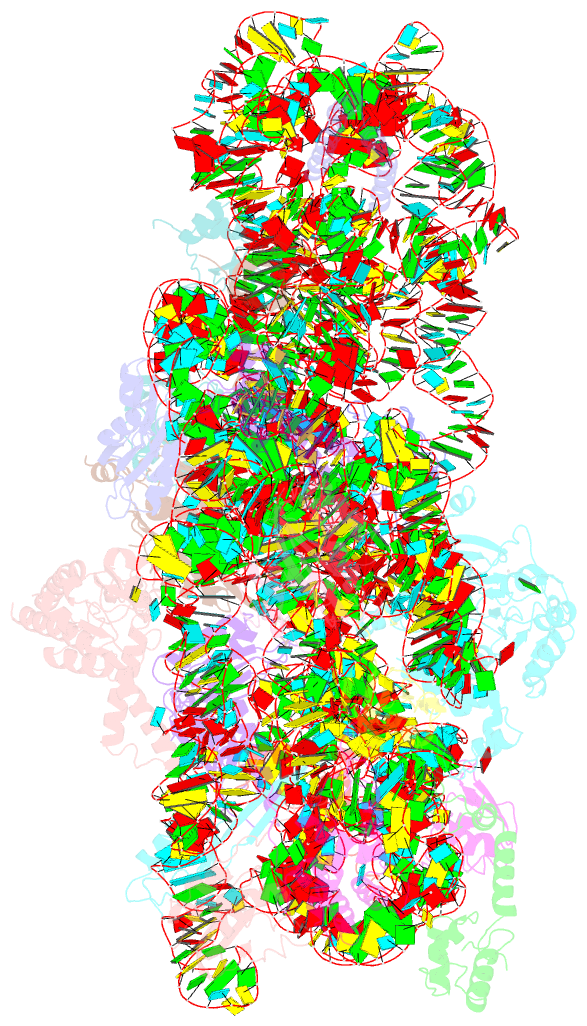
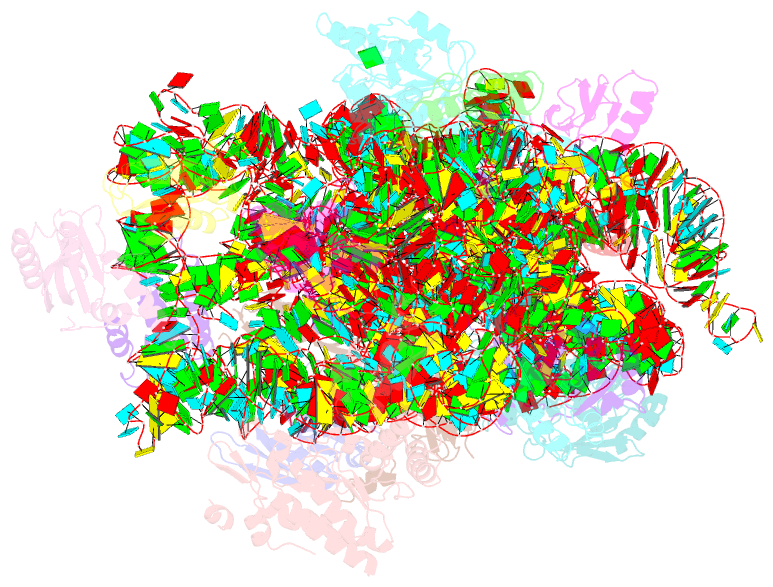
Summary information and primary citation
- PDB-id
- 5uz4; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ribosome-hydrolase
- Method
- cryo-EM (5.8 Å)
- Summary
- The cryo-EM structure of yjeq bound to the 30s subunit suggests a fidelity checkpoint function for this protein in ribosome assembly
- Reference
- Razi A, Guarne A, Ortega J (2017): "The cryo-EM structure of YjeQ bound to the 30S subunit suggests a fidelity checkpoint function for this protein in ribosome assembly." Proc. Natl. Acad. Sci. U.S.A., 114, E3396-E3403. doi: 10.1073/pnas.1618016114.
- Abstract
- Recent work suggests that bacterial YjeQ (RsgA) participates in the late stages of assembly of the 30S subunit and aids the assembly of the decoding center but also binds the mature 30S subunit with high affinity. To determine the function and mechanisms of YjeQ in the context of the mature subunit, we determined the cryo-EM structure of the fully assembled 30S subunit in complex with YjeQ at 5.8-Å resolution. We found that binding of YjeQ stabilizes helix 44 into a conformation similar to that adopted by the subunit during proofreading. This finding indicates that, along with acting as an assembly factor, YjeQ has a role as a checkpoint protein, consisting of testing the proofreading ability of the 30S subunit. The structure also informs the mechanism by which YjeQ implements the release from the 30S subunit of a second assembly factor, called RbfA. Finally, it reveals how the 30S subunit stimulates YjeQ GTPase activity and leads to release of the protein. Checkpoint functions have been described for eukaryotic ribosome assembly factors; however, this work describes an example of a bacterial assembly factor that tests a specific translation mechanism of the 30S subunit.