Summary information and primary citation
- PDB-id
- 5wqe; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein
- Method
- X-ray (3.126 Å)
- Summary
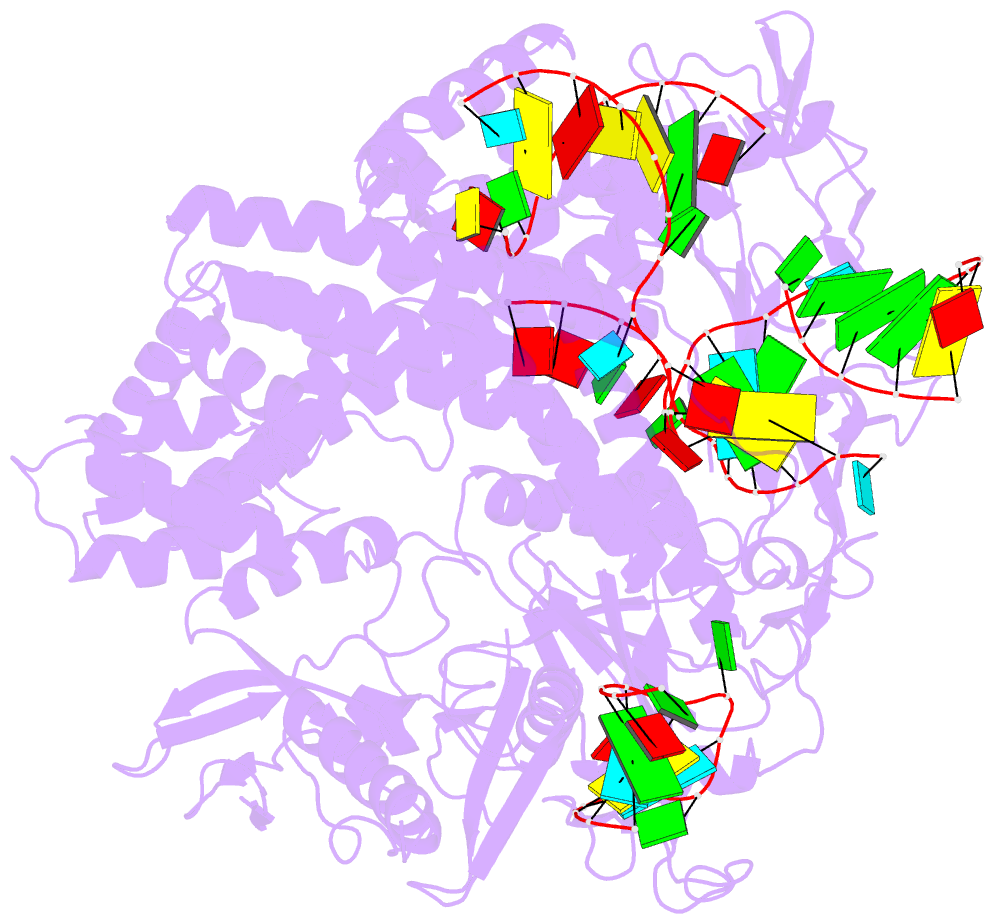
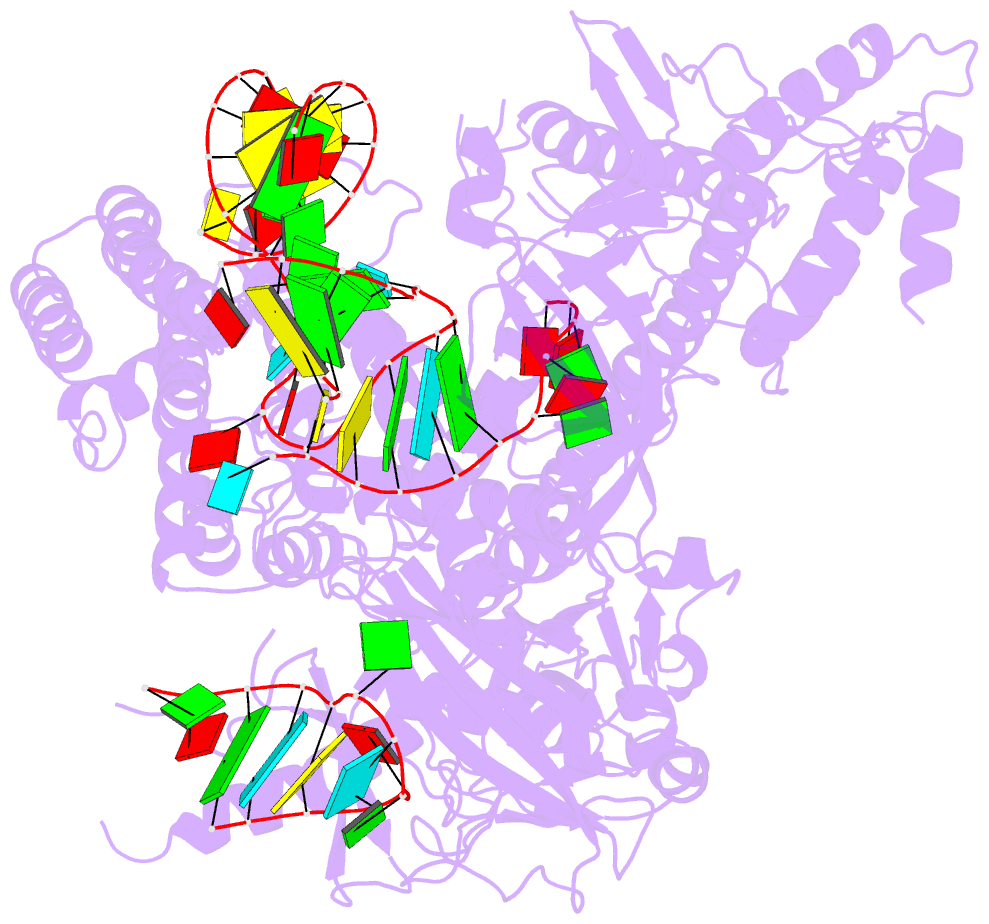
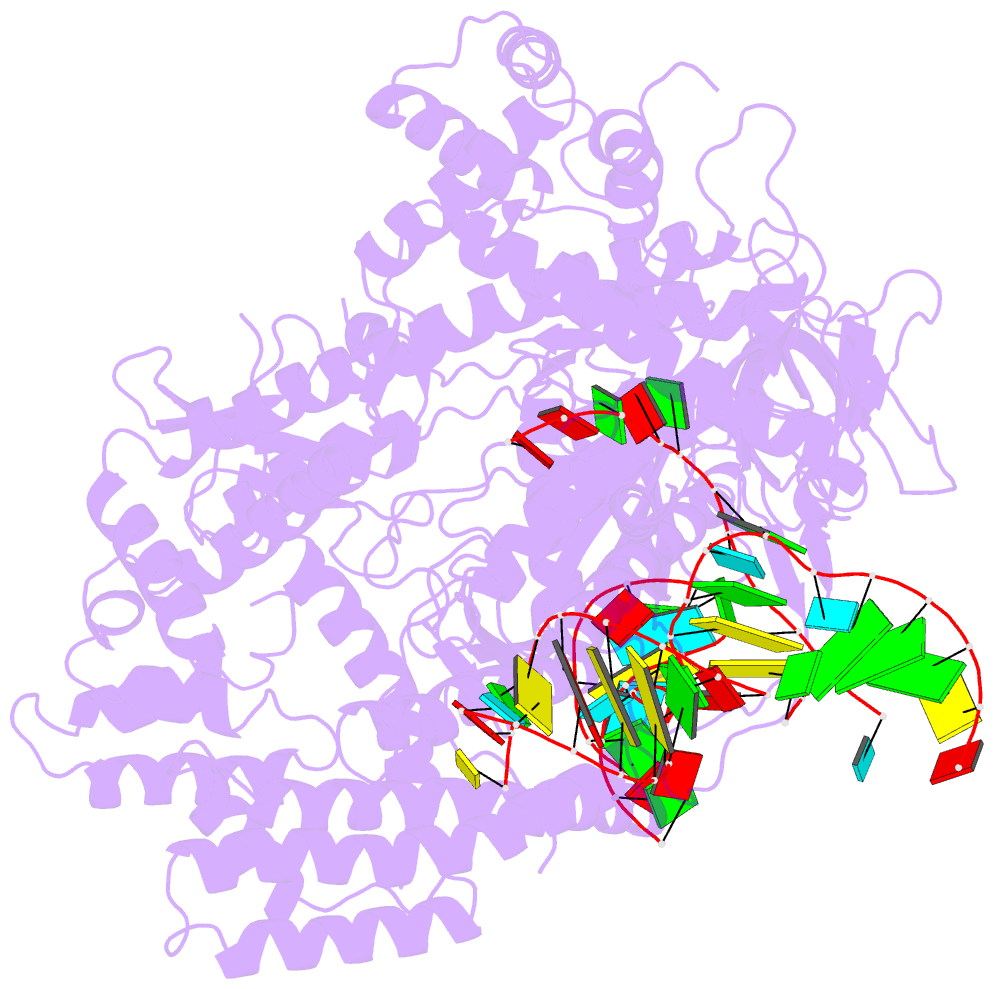
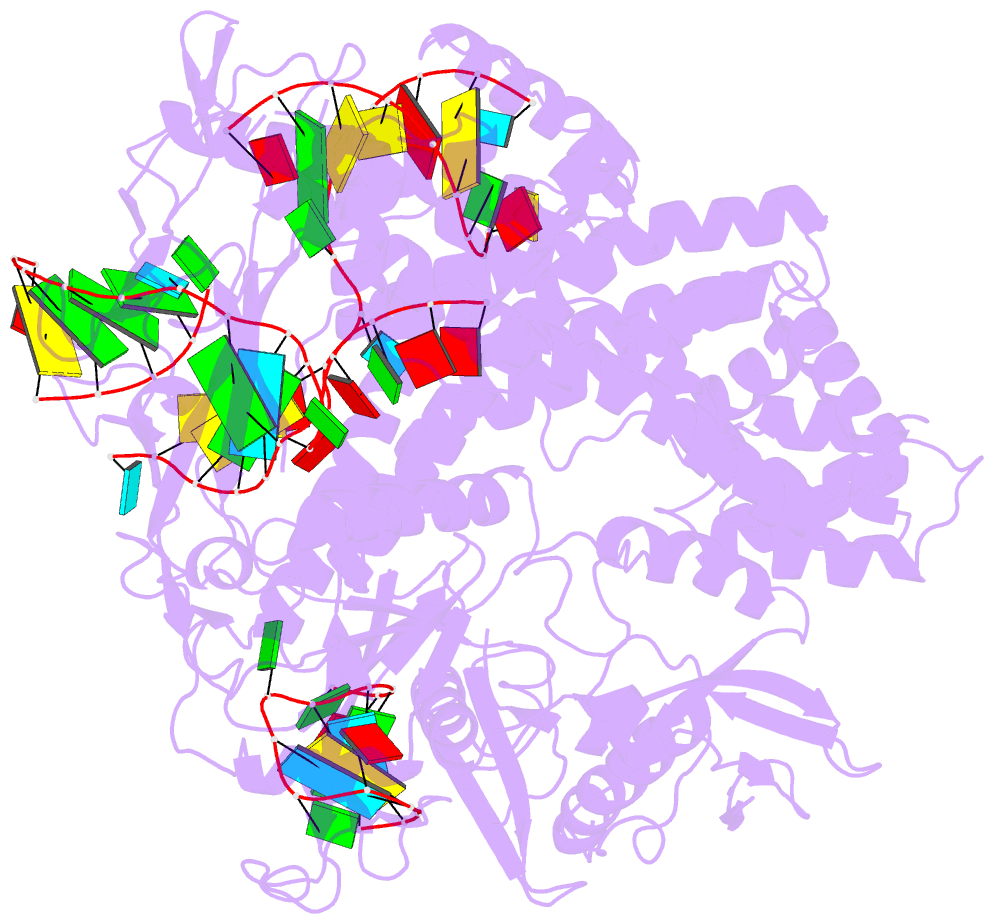
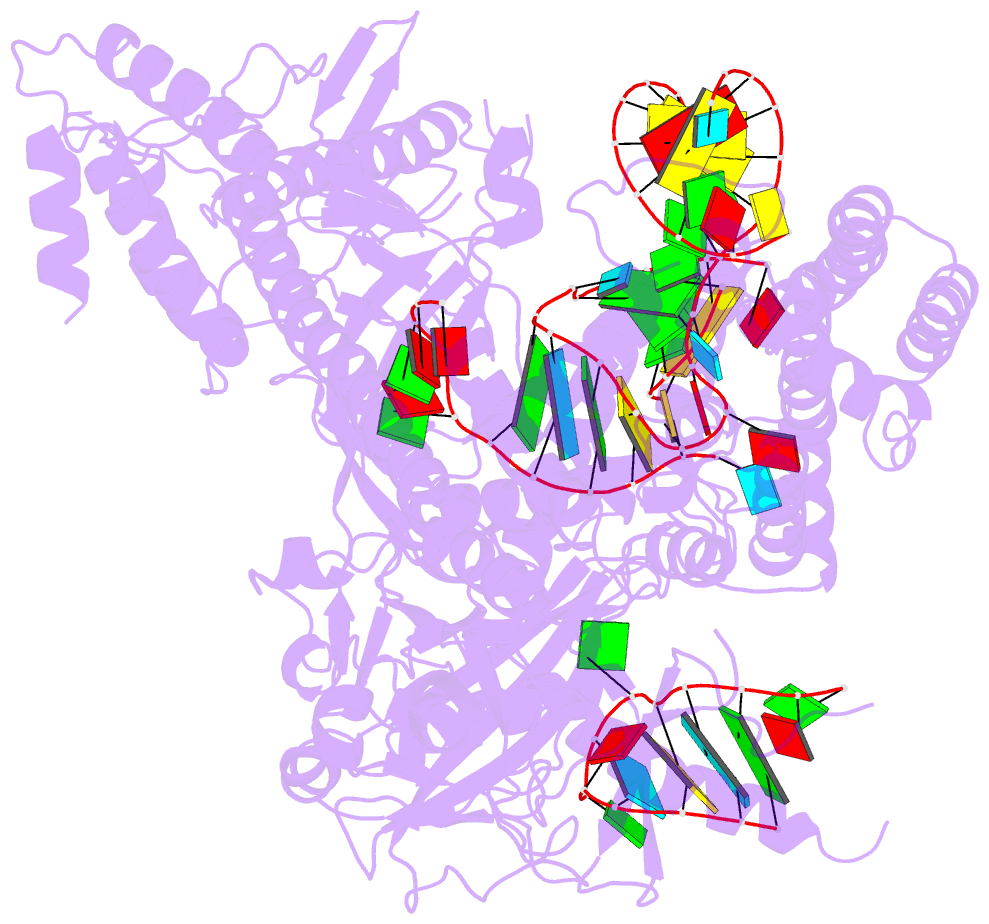
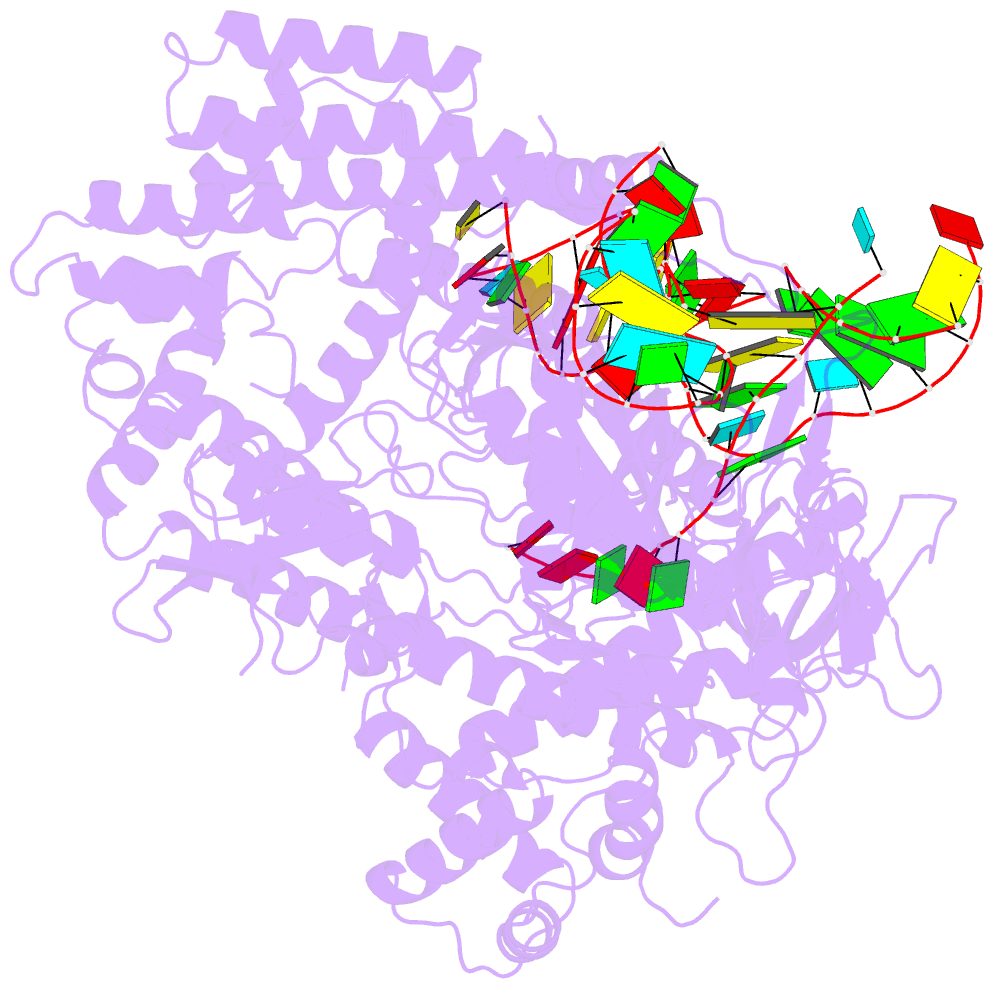
- Crystal structure of alicyclobacillus acidoterrestris c2c1 in complex with single-guide RNA at 3.1 angstrom resolution
- Reference
- Liu L, Chen P, Wang M, Li X, Wang J, Yin M, Wang Y (2017): "C2c1-sgRNA Complex Structure Reveals RNA-Guided DNA Cleavage Mechanism." Mol. Cell, 65, 310-322. doi: 10.1016/j.molcel.2016.11.040.
- Abstract
- C2c1 is a type V-B CRISPR-Cas system dual-RNA-guided DNA endonuclease. Here, we report the crystal structure of Alicyclobacillus acidoterrestris C2c1 in complex with a chimeric single-molecule guide RNA (sgRNA). AacC2c1 exhibits a bi-lobed architecture consisting of a REC and NUC lobe. The sgRNA scaffold forms a tetra-helical structure, distinct from previous predictions. The crRNA is located in the central channel of C2c1, and the tracrRNA resides in an external surface groove. Although AacC2c1 lacks a PAM-interacting domain, our analysis revealed that the PAM duplex has a similar binding position found in Cpf1. Importantly, C2c1-sgRNA system is highly sensitive to single-nucleotide mismatches between guide RNA and target DNA. The resulting reduction in off-target cleavage renders C2c1 a valuable addition to the current arsenal of genome-editing tools. Together, our findings indicate that sgRNA assembly is achieved through a mechanism distinct from that reported previously for Cas9 or Cpf1 endonucleases.