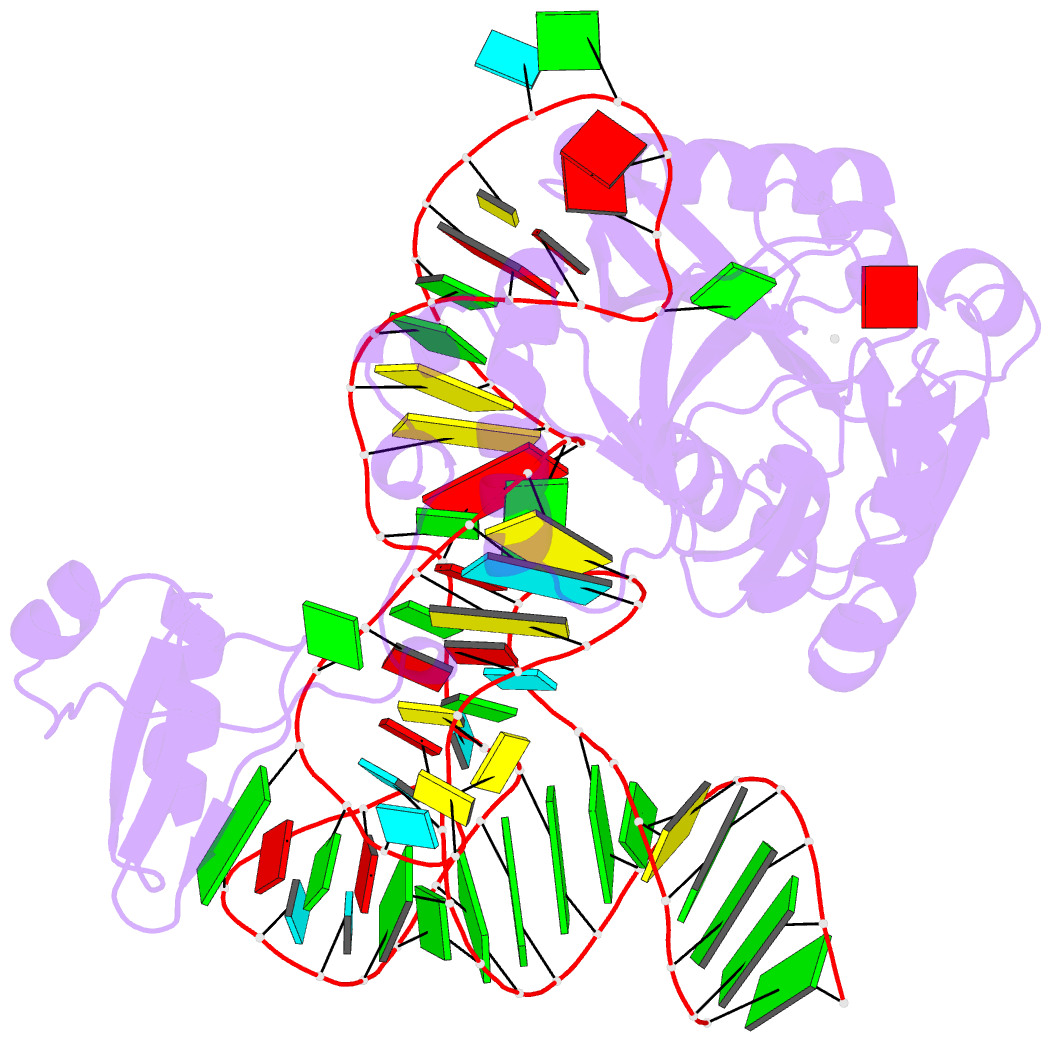
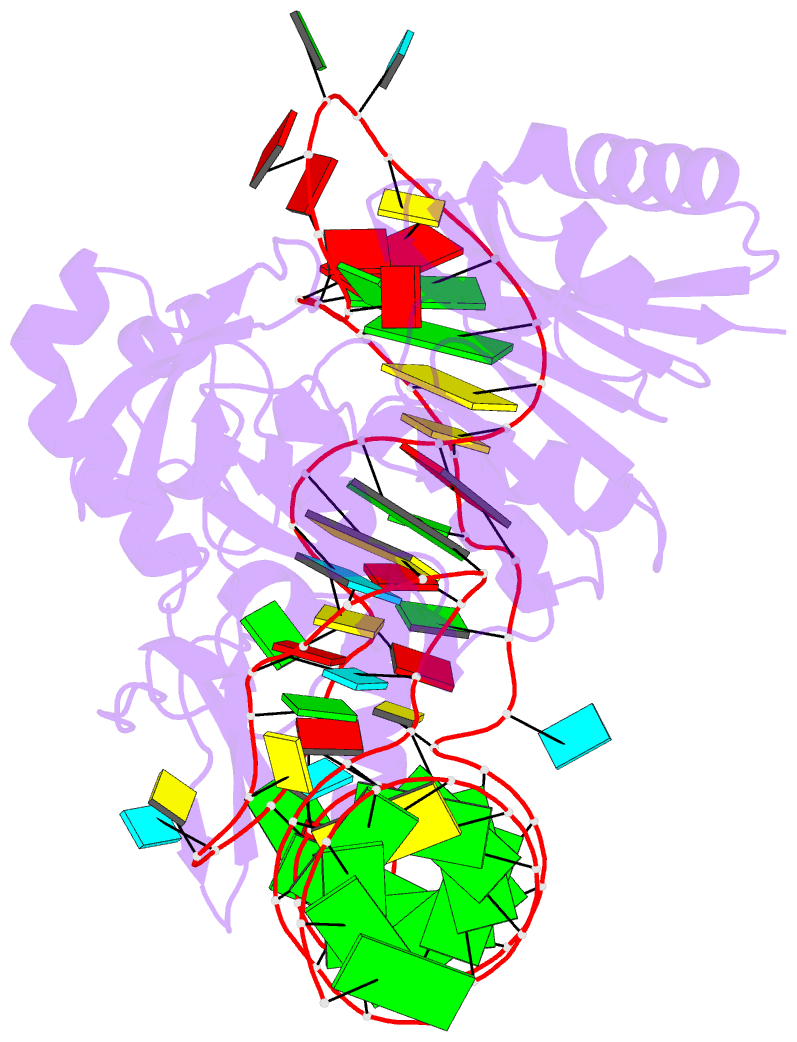
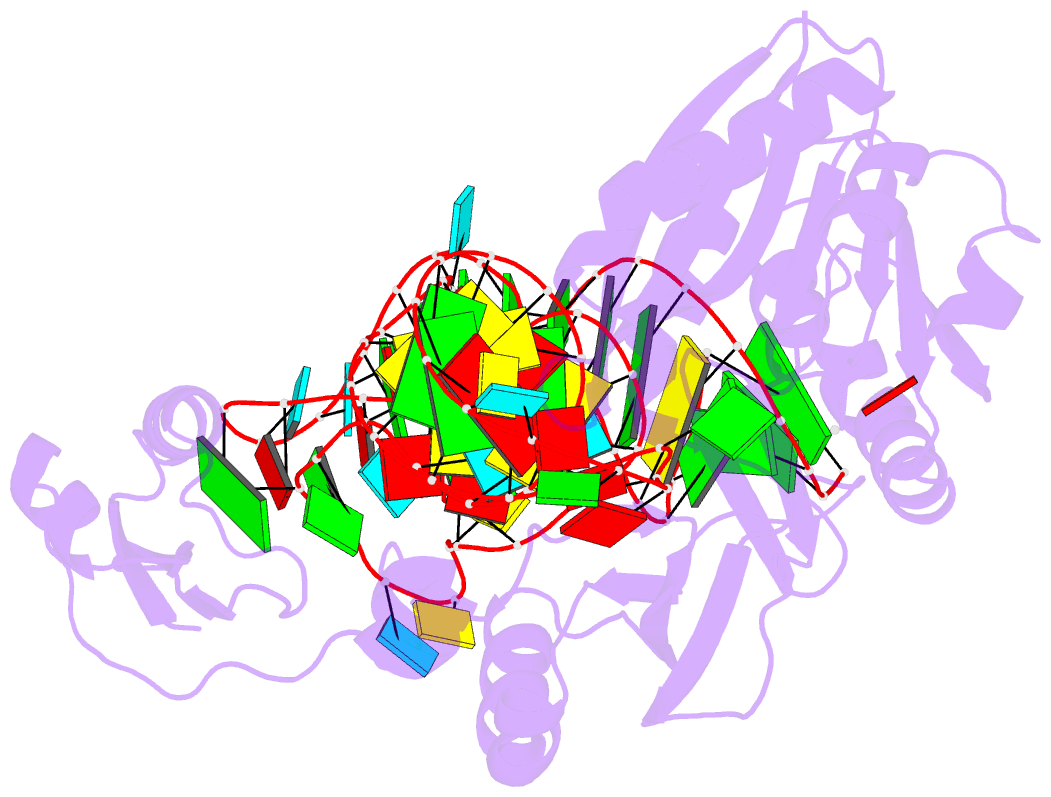
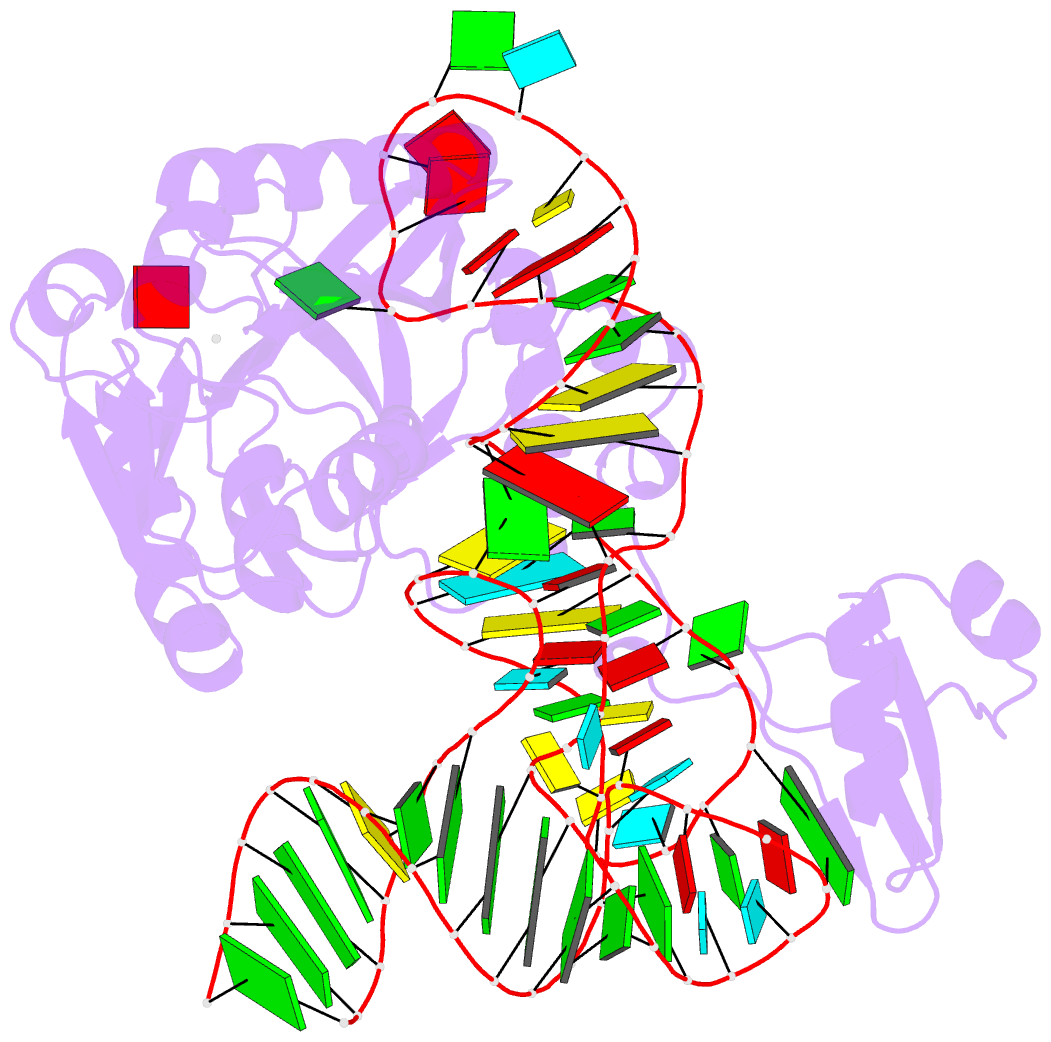
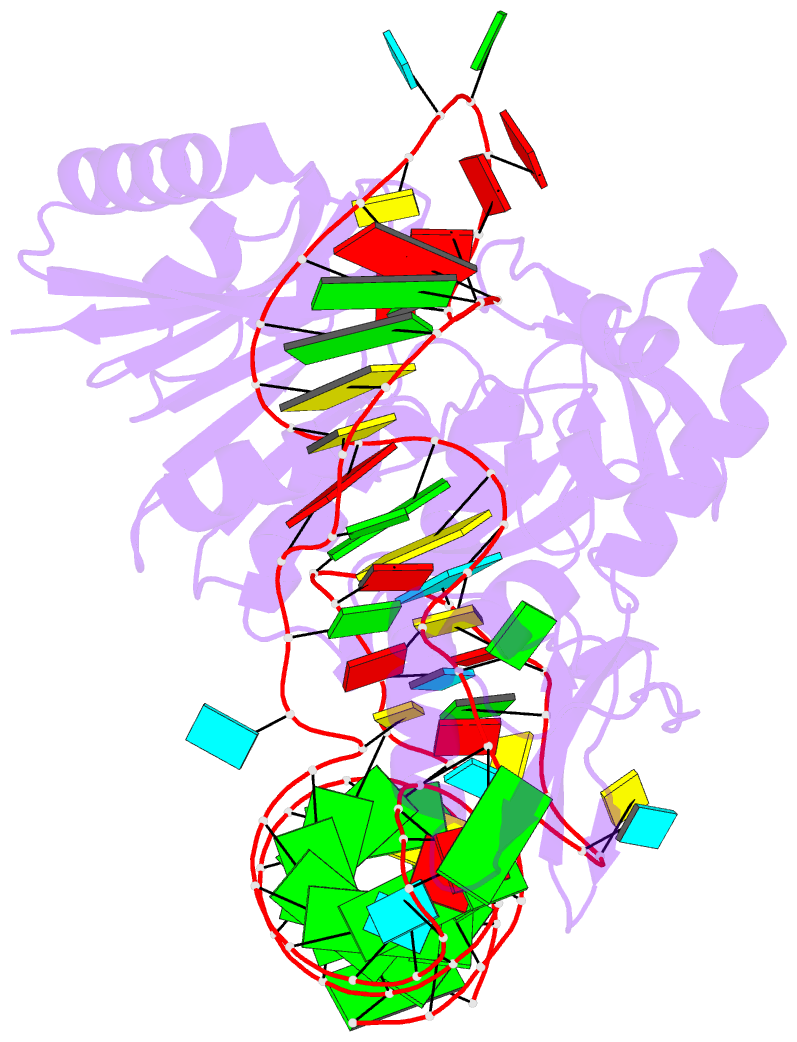
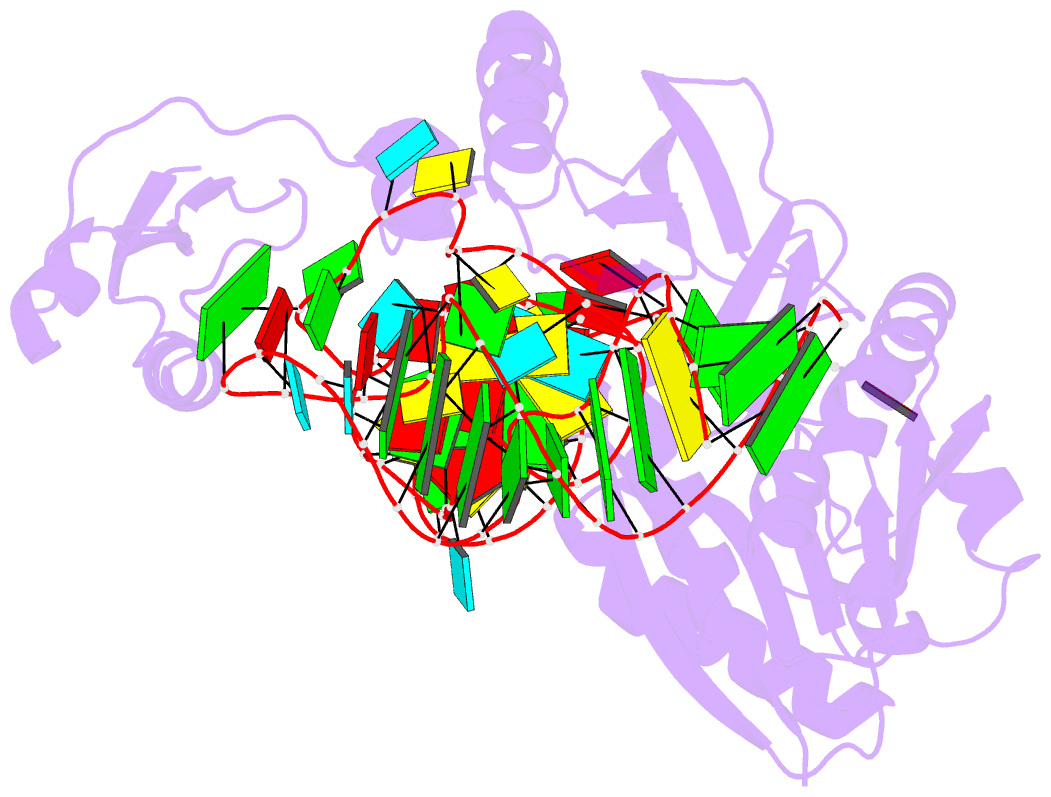
Summary information and primary citation
- PDB-id
- 5wt3; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase-RNA
- Method
- X-ray (3.204 Å)
- Summary
- Pyrococcus abyssi methyltransferase patrm5a bound by mta and cognate trna
- Reference
- Wang C, Jia Q, Zeng J, Chen R, Xie W (2017): "Structural insight into the methyltransfer mechanism of the bifunctional Trm5." Sci Adv, 3, e1700195. doi: 10.1126/sciadv.1700195.
- Abstract
- The wyosine derivatives present at position 37 in transfer RNAs (tRNAs) are critical for reading frame maintenance. The methyltransferase Trm5a from Pyrococcus abyssi (PaTrm5a) plays a key role in this hypermodification process in generating m1G37 and imG2, two products of the wyosine biosynthetic pathway, through two methyl transfers to distinct substrates, but the mechanism is currently unknown. We report two cocrystal structures of PaTrm5a in complex with tRNAPhe and reveal the structural basis for substrate recognition, which was supported by in vitro activity assays. The crystal structures showed that the D1 domain of the enzyme undergoes large conformational changes upon the binding of tRNA. The deletion of this domain greatly reduces the affinity and activity of PaTrm5a toward its RNA substrate, indicating that the enzyme recognizes the overall shape of tRNA. Using the small-angle x-ray scattering technique and crystallographic analysis, we discovered that PaTrm5a adopts distinct open conformations before and after the binding of tRNA. Last, through structure comparison with its ortholog Methanococcus jannaschii Trm5b (MjTrm5b), we propose a reaction mechanism for the double methylation capability of this unique enzyme.