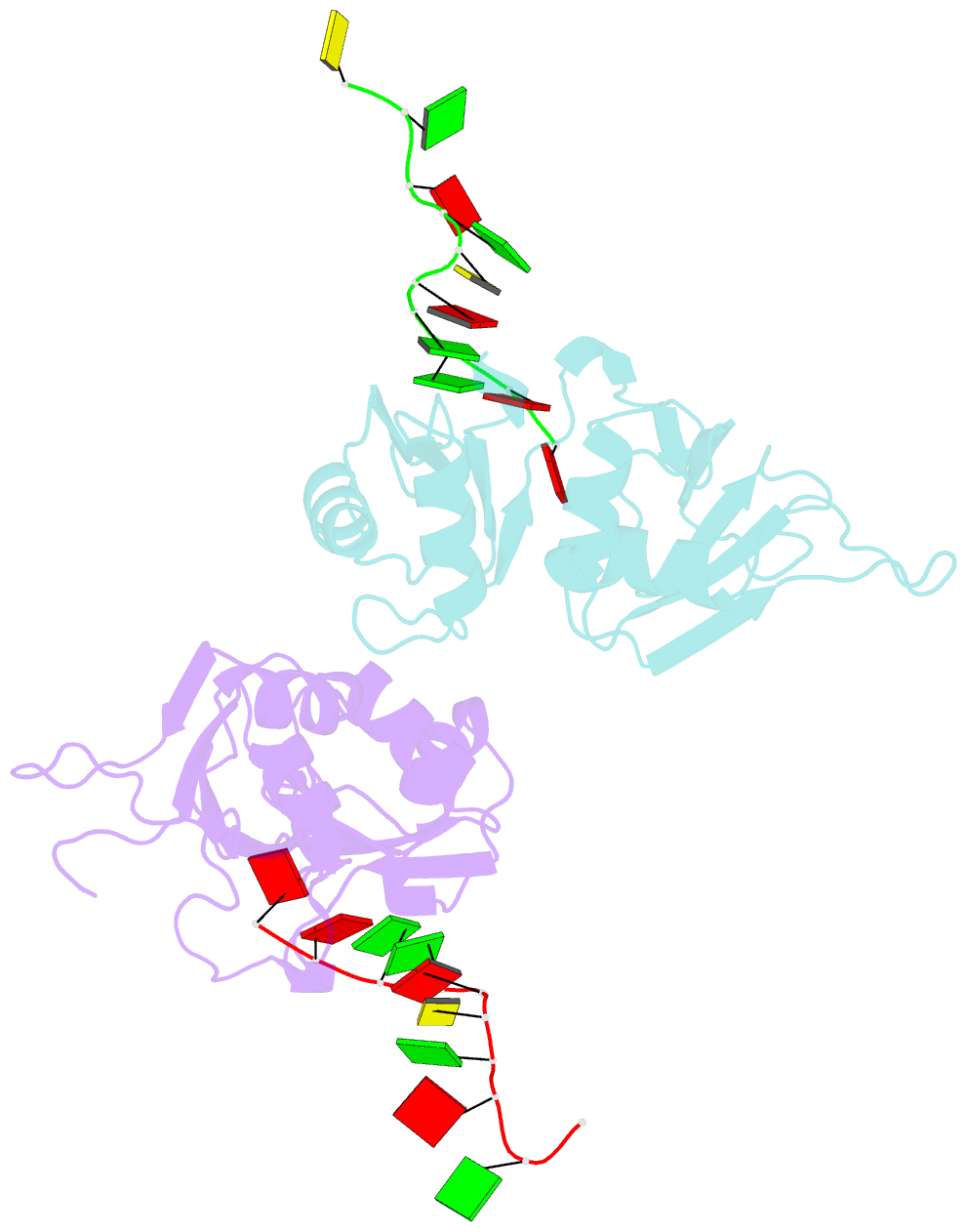
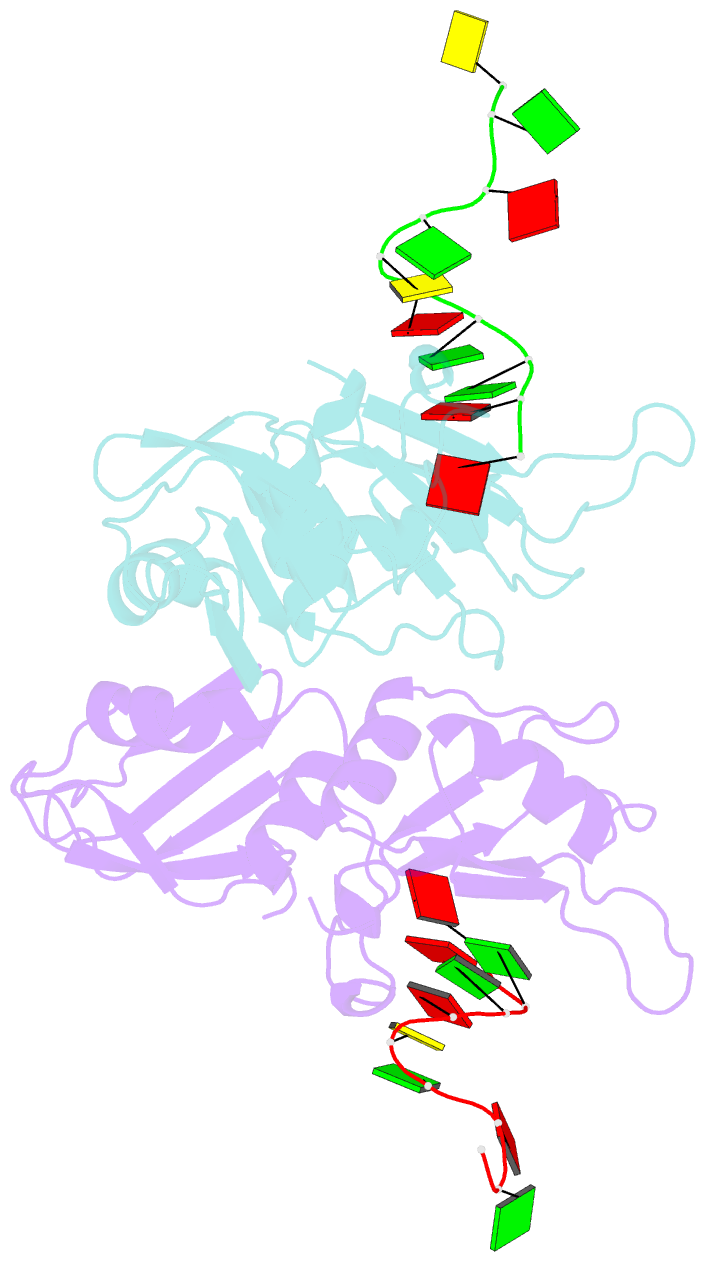
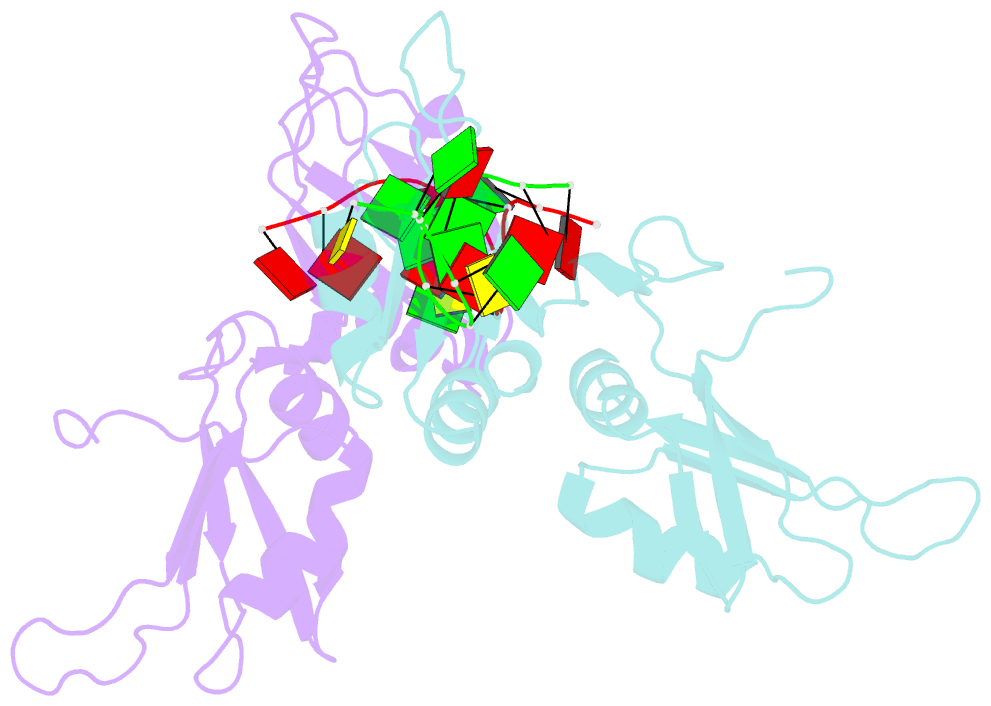
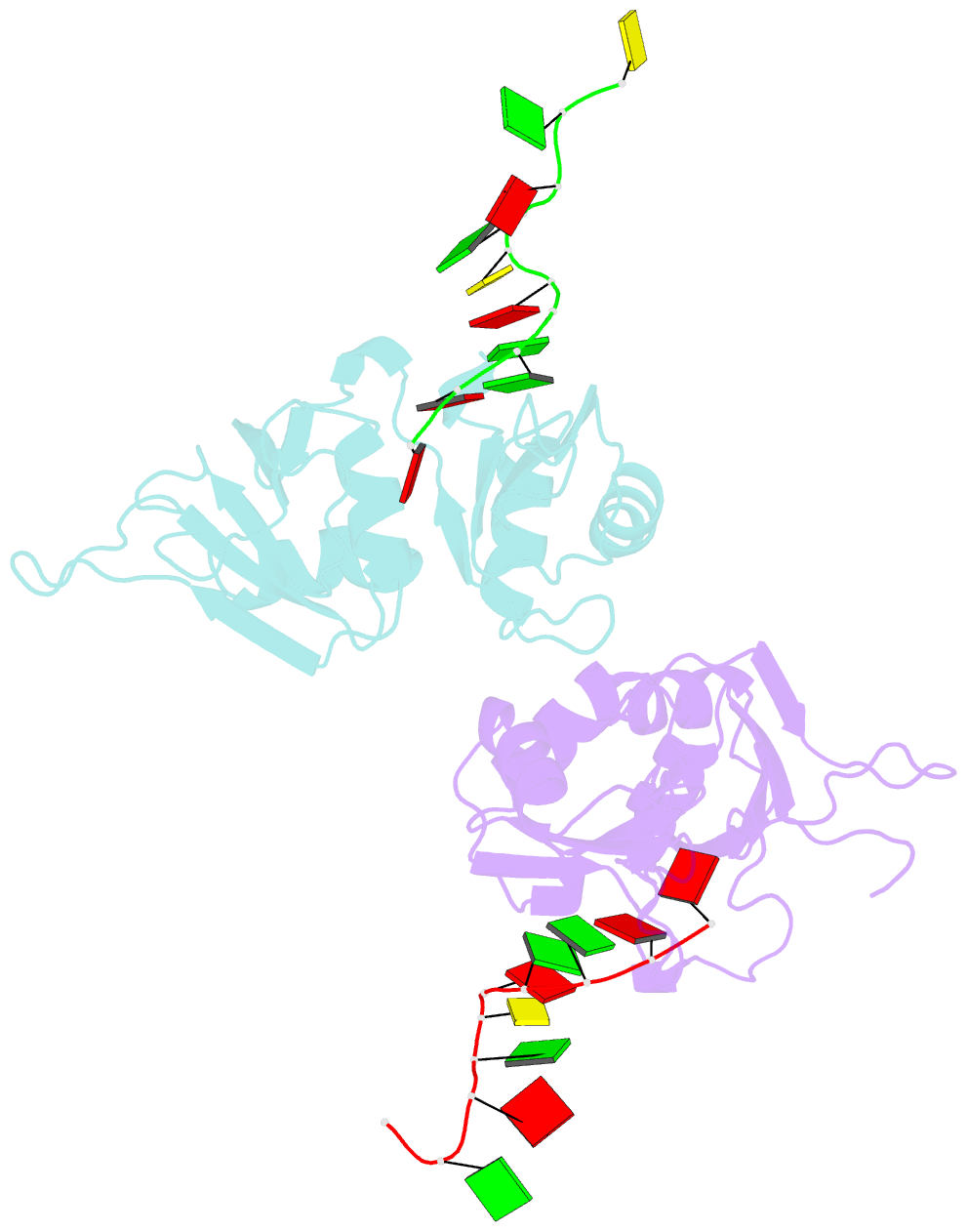
Summary information and primary citation
- PDB-id
- 5wwf; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein-RNA
- Method
- X-ray (2.15 Å)
- Summary
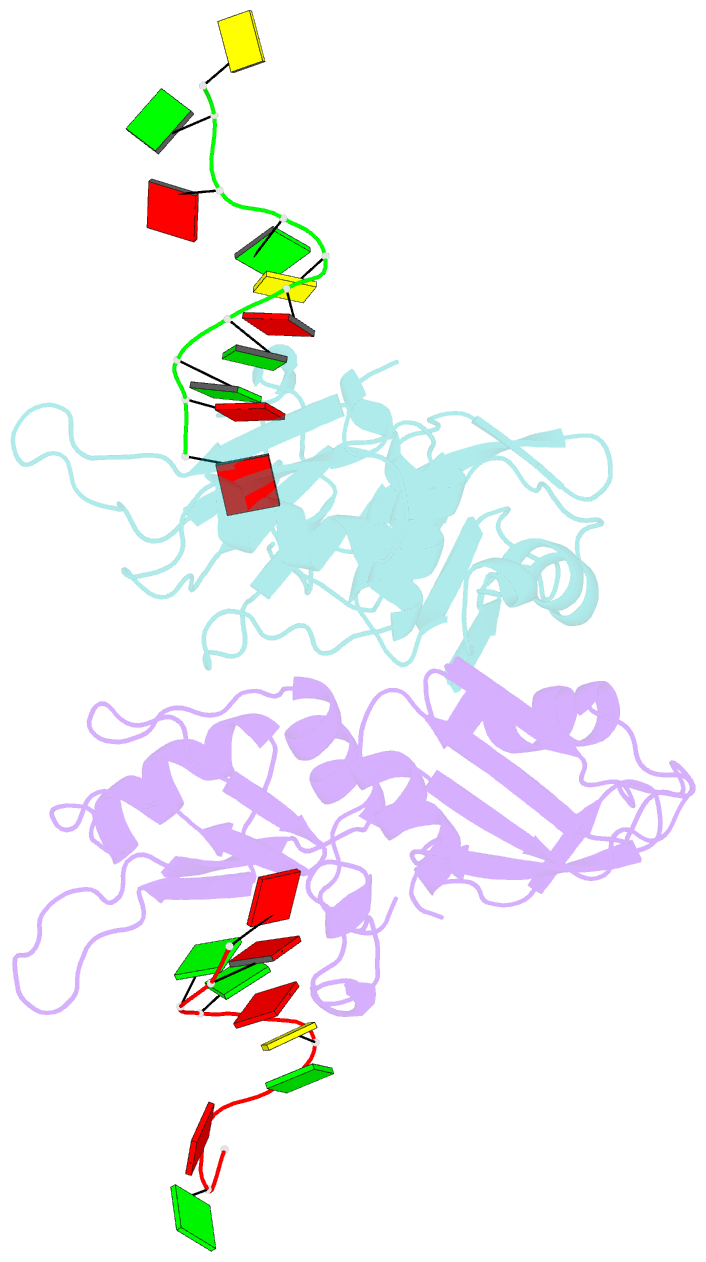
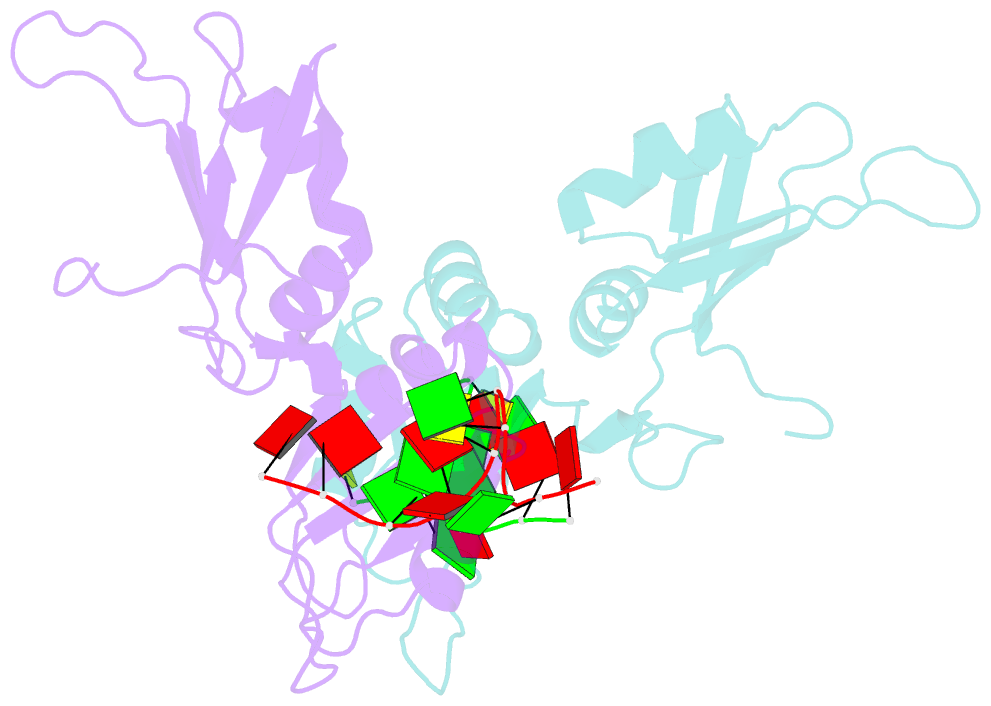
- Crystal structure of hnrnpa2b1 in complex with RNA
- Reference
- Wu B, Su S, Patil DP, Liu H, Gan J, Jaffrey SR, Ma J (2018): "Molecular basis for the specific and multivariant recognitions of RNA substrates by human hnRNP A2/B1." Nat Commun, 9, 420. doi: 10.1038/s41467-017-02770-z.
- Abstract
- Human hnRNP A2/B1 is an RNA-binding protein that plays important roles in many biological processes, including maturation, transport, and metabolism of mRNA, and gene regulation of long noncoding RNAs. hnRNP A2/B1 was reported to control the microRNAs sorting to exosomes and promote primary microRNA processing as a potential m6A "reader." hnRNP A2/B1 contains two RNA recognition motifs that provide sequence-specific recognition of RNA substrates. Here, we determine crystal structures of tandem RRM domains of hnRNP A2/B1 in complex with various RNA substrates, elucidating specific recognitions of AGG and UAG motifs by RRM1 and RRM2 domains, respectively. Further structural and biochemical results demonstrate multivariant binding modes for sequence-diversified RNA substrates, supporting a RNA matchmaker mechanism in hnRNP A2/B1 function. Moreover, our studies in combination with bioinformatic analysis suggest that hnRNP A2/B1 may mediate effects of m6A through a "m6A switch" mechanism, instead of acting as a direct "reader" of m6A modification.