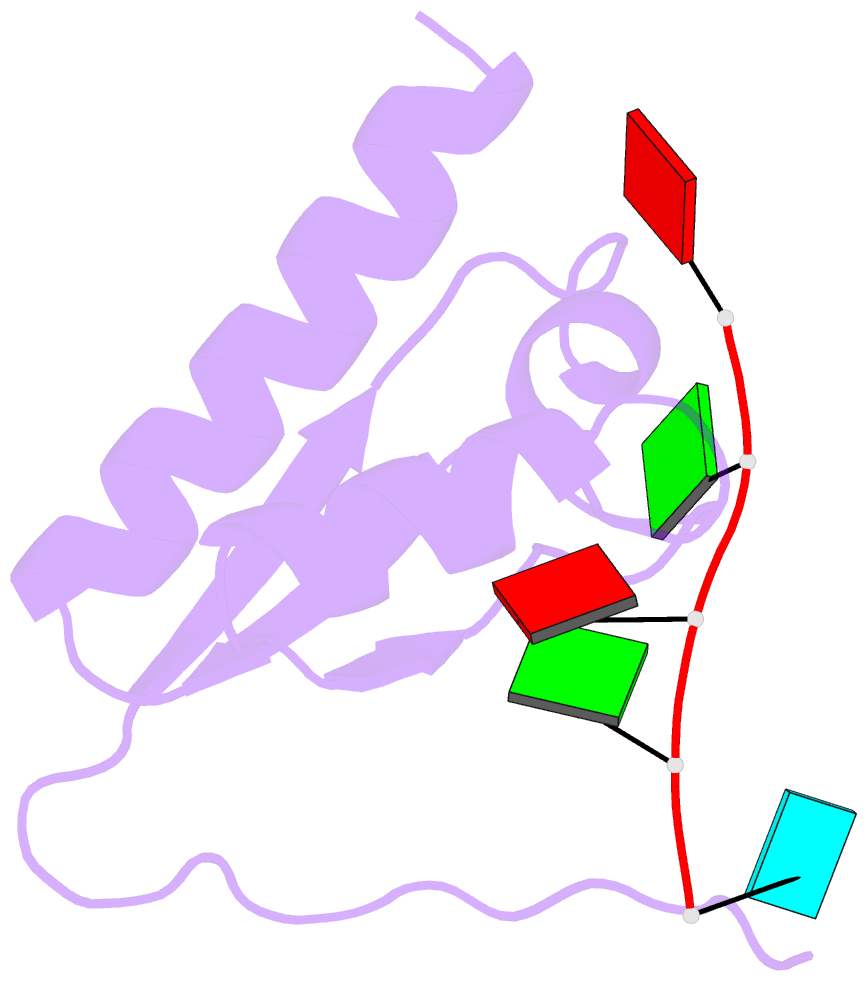
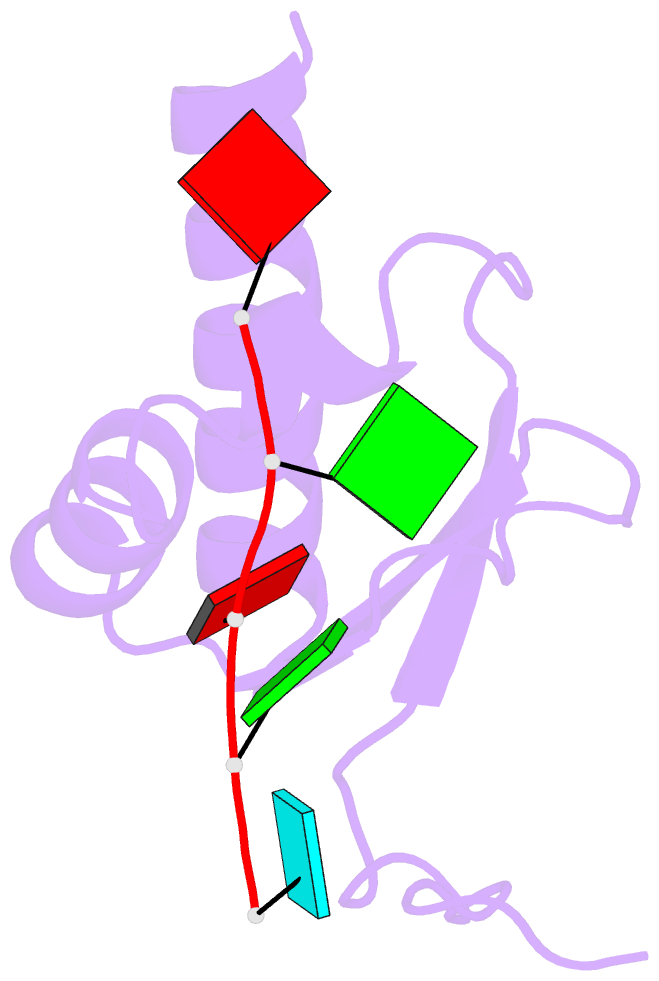
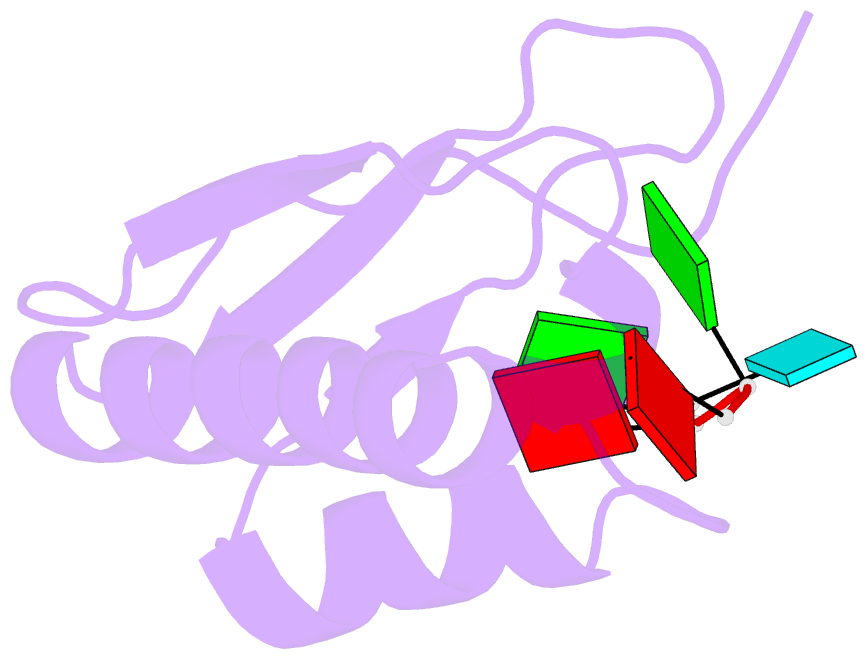
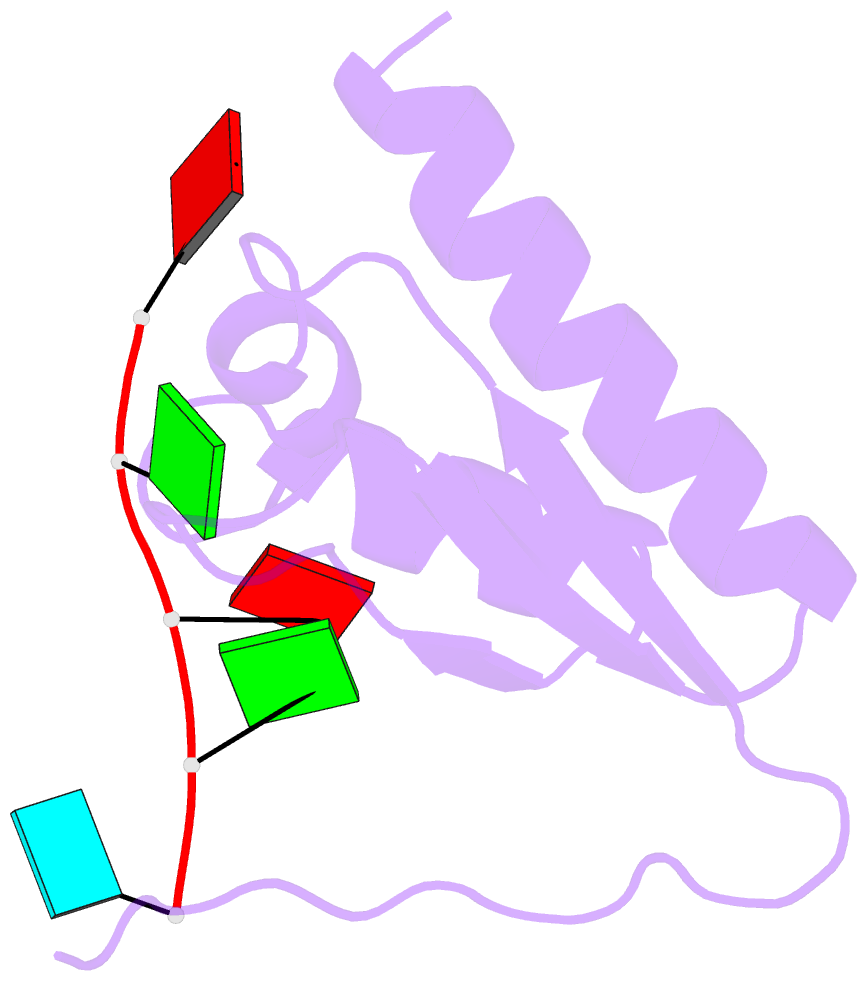
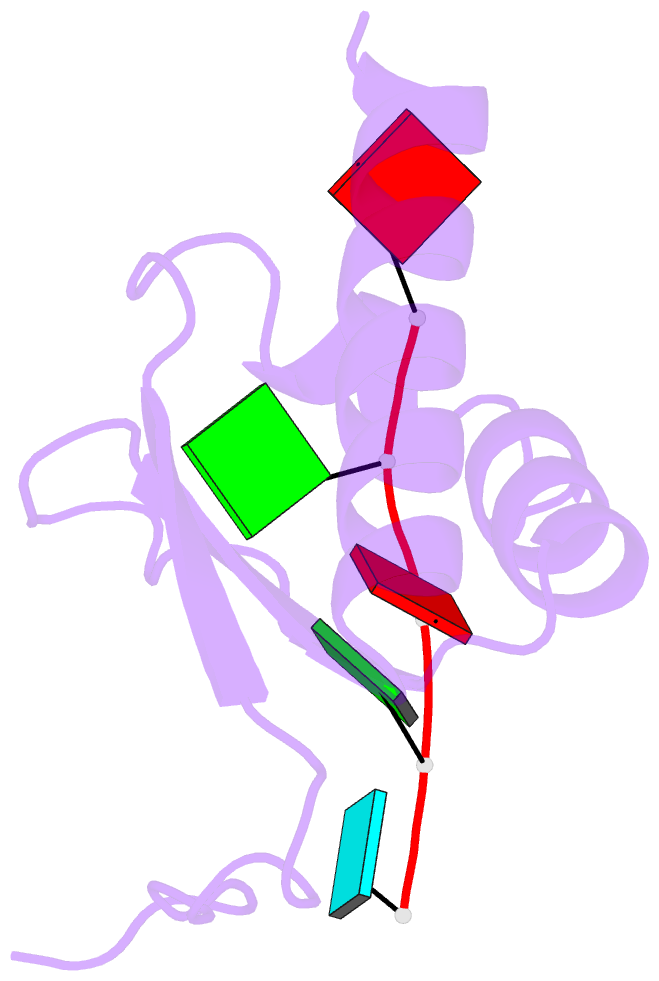
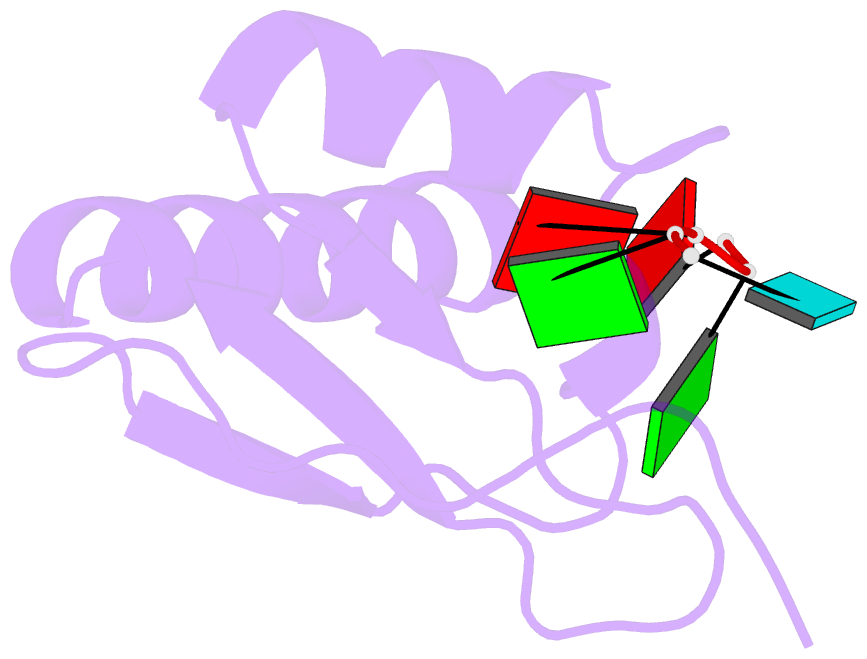
Summary information and primary citation
- PDB-id
- 5wwx; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein-RNA
- Method
- X-ray (2.0 Å)
- Summary
- Crystal structure of the kh2 domain of human RNA-binding e3 ubiquitin-protein ligase mex-3c complex with RNA
- Reference
- Yang L, Wang C, Li F, Zhang J, Nayab A, Wu J, Shi Y, Gong Q (2017): "The human RNA-binding protein and E3 ligase MEX-3C binds the MEX-3-recognition element (MRE) motif with high affinity." J. Biol. Chem., 292, 16221-16234. doi: 10.1074/jbc.M117.797746.
- Abstract
- MEX-3 is a K-homology (KH) domain-containing RNA-binding protein first identified as a translational repressor in Caenorhabditis elegans, and its four orthologs (MEX-3A-D) in human and mouse were subsequently found to have E3 ubiquitin ligase activity mediated by a RING domain and critical for RNA degradation. Current evidence implicates human MEX-3C in many essential biological processes and suggests a strong connection with immune diseases and carcinogenesis. The highly conserved dual KH domains in MEX-3 proteins enable RNA binding and are essential for the recognition of the 3'-UTR and post-transcriptional regulation of MEX-3 target transcripts. However, the molecular mechanisms of translational repression and the consensus RNA sequence recognized by the MEX-3C KH domain are unknown. Here, using X-ray crystallography and isothermal titration calorimetry, we investigated the RNA-binding activity and selectivity of human MEX-3C dual KH domains. Our high-resolution crystal structures of individual KH domains complexed with a noncanonical U-rich and a GA-rich RNA sequence revealed that the KH1/2 domains of human MEX-3C bound MRE10, a 10-mer RNA (5'-CAGAGUUUAG-3') consisting of an eight-nucleotide MEX-3-recognition element (MRE) motif, with high affinity. Of note, we also identified a consensus RNA motif recognized by human MEX-3C. The potential RNA-binding sites in the 3'-UTR of the human leukocyte antigen serotype (HLA-A2) mRNA were mapped with this RNA-binding motif and further confirmed by fluorescence polarization. The binding motif identified here will provide valuable information for future investigations of the functional pathways controlled by human MEX-3C and for predicting potential mRNAs regulated by this enzyme.