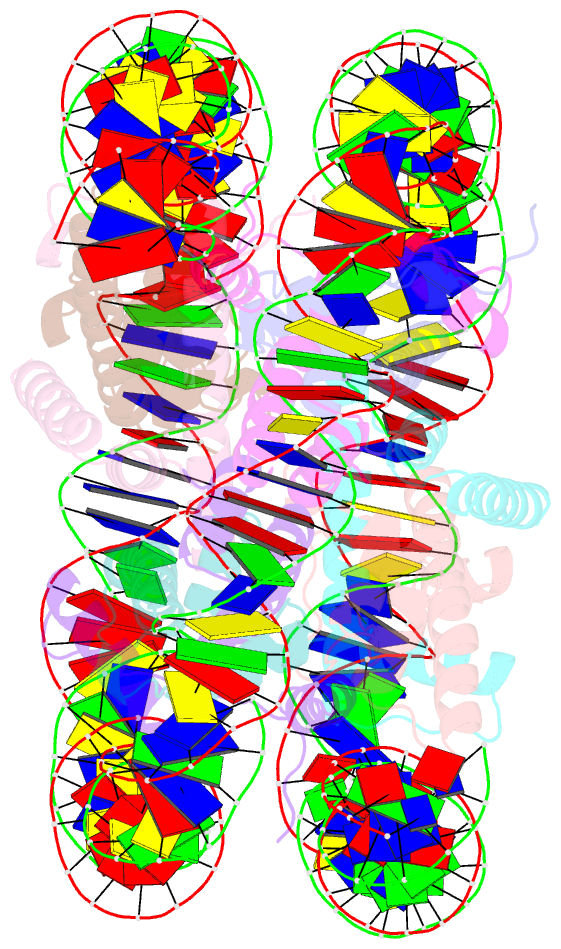
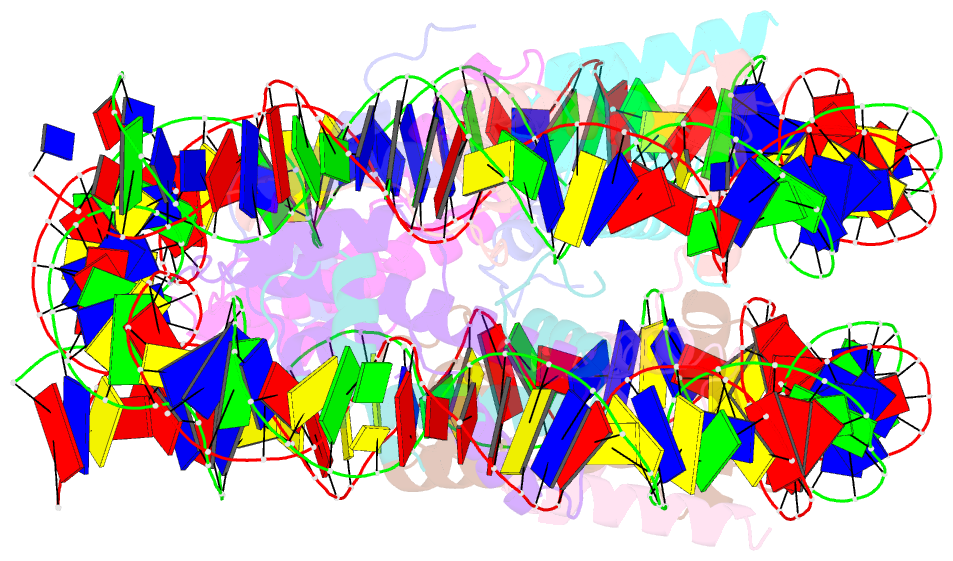
Summary information and primary citation
- PDB-id
- 5xm0; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- structural protein-DNA
- Method
- X-ray (2.874 Å)
- Summary
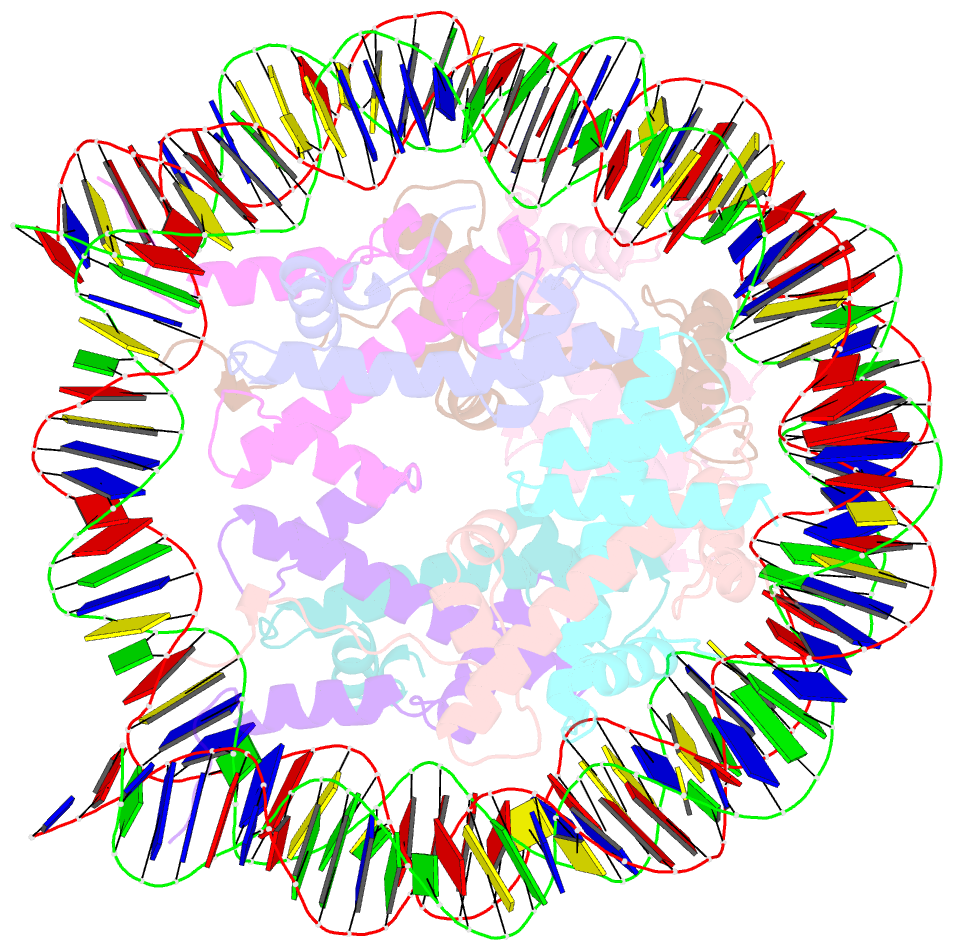
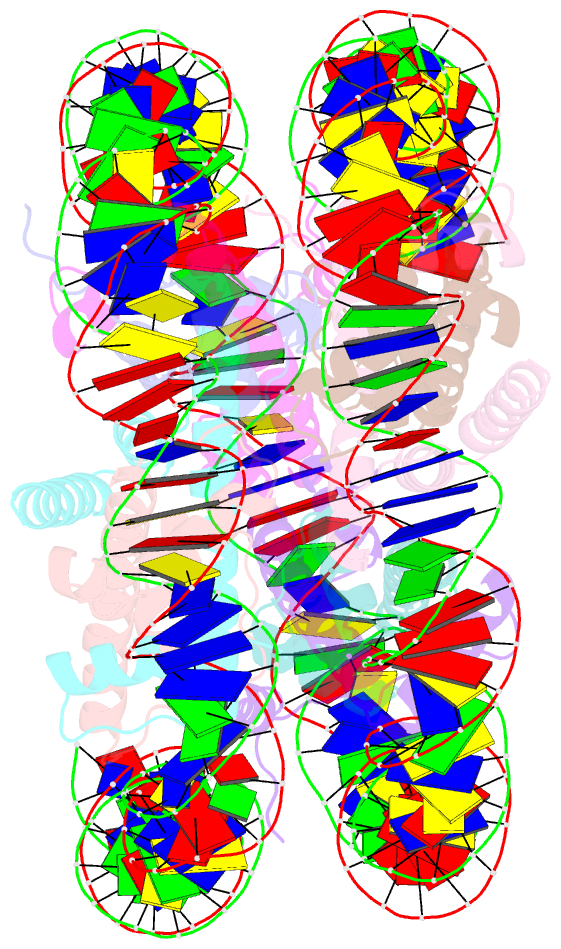
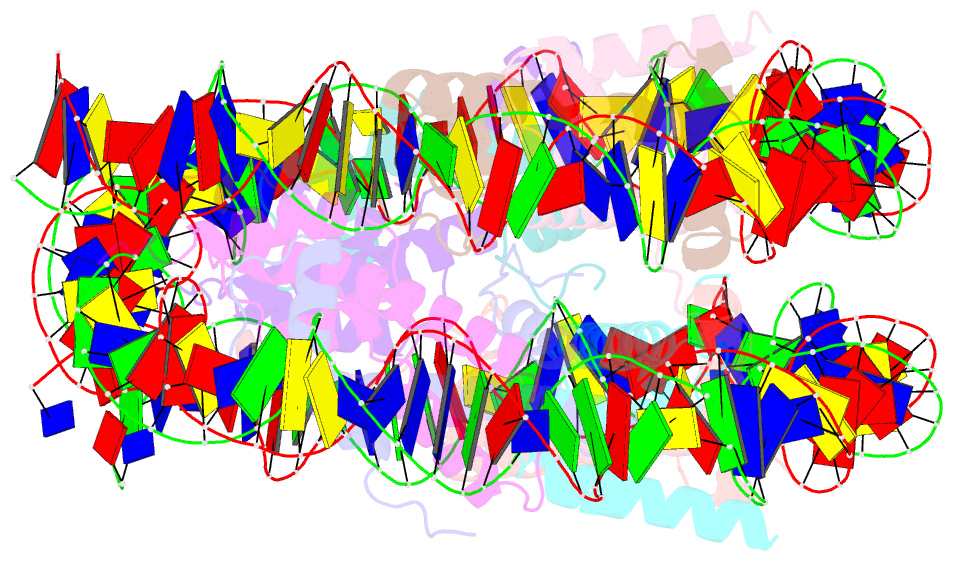
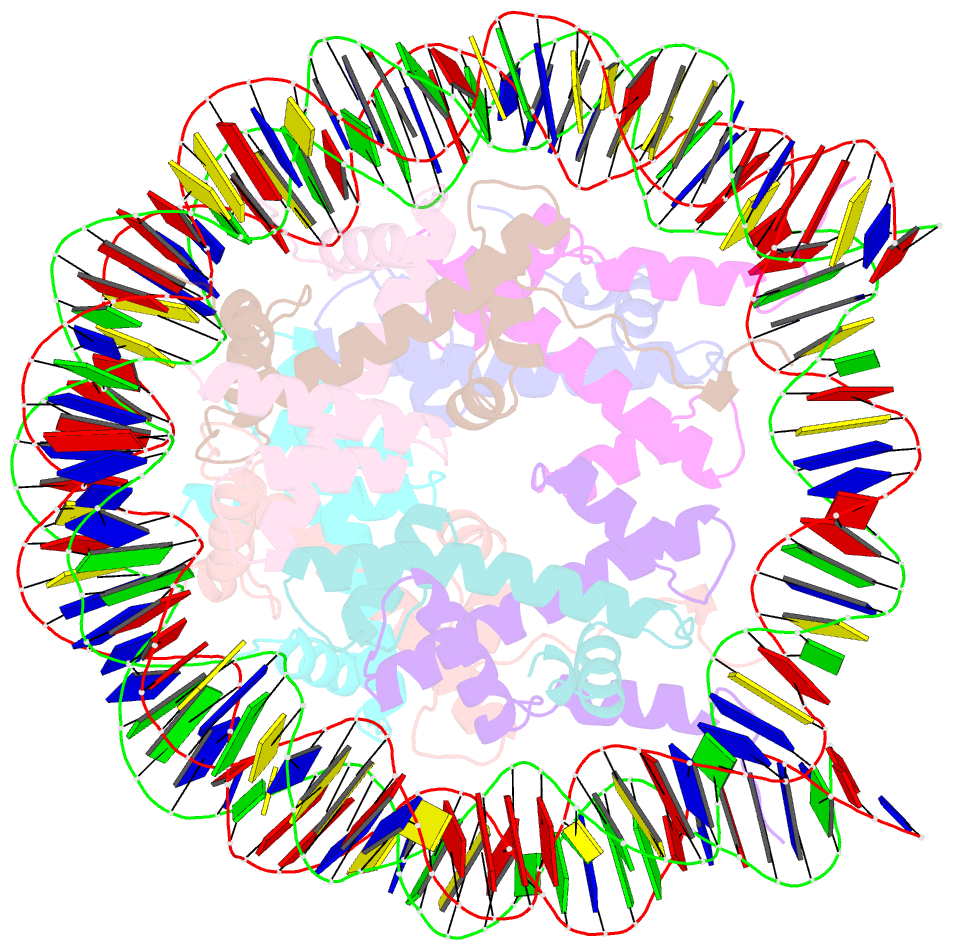
- The mouse nucleosome structure containing h2a, h2b type3-a, h3.3, and h4
- Reference
- Harada A, Maehara K, Ono Y, Taguchi H, Yoshioka K, Kitajima Y, Xie Y, Sato Y, Iwasaki T, Nogami J, Okada S, Komatsu T, Semba Y, Takemoto T, Kimura H, Kurumizaka H, Ohkawa Y (2018): "Histone H3.3 sub-variant H3mm7 is required for normal skeletal muscle regeneration." Nat Commun, 9, 1400. doi: 10.1038/s41467-018-03845-1.
- Abstract
- Regulation of gene expression requires selective incorporation of histone H3 variant H3.3 into chromatin. Histone H3.3 has several subsidiary variants but their functions are unclear. Here we characterize the function of histone H3.3 sub-variant, H3mm7, which is expressed in skeletal muscle satellite cells. H3mm7 knockout mice demonstrate an essential role of H3mm7 in skeletal muscle regeneration. Chromatin analysis reveals that H3mm7 facilitates transcription by forming an open chromatin structure around promoter regions including those of myogenic genes. The crystal structure of the nucleosome containing H3mm7 reveals that, unlike the S57 residue of other H3 proteins, the H3mm7-specific A57 residue cannot form a hydrogen bond with the R40 residue of the cognate H4 molecule. Consequently, the H3mm7 nucleosome is unstable in vitro and exhibited higher mobility in vivo compared with the H3.3 nucleosome. We conclude that the unstable H3mm7 nucleosome may be required for proper skeletal muscle differentiation.