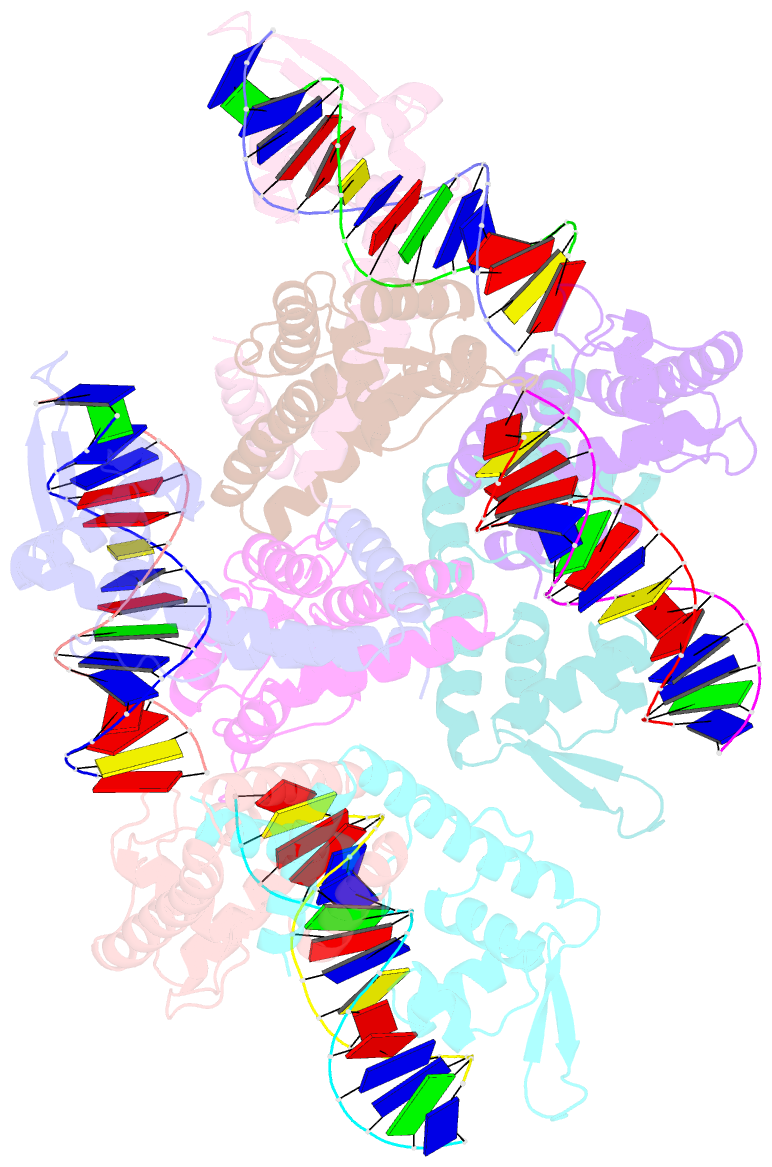
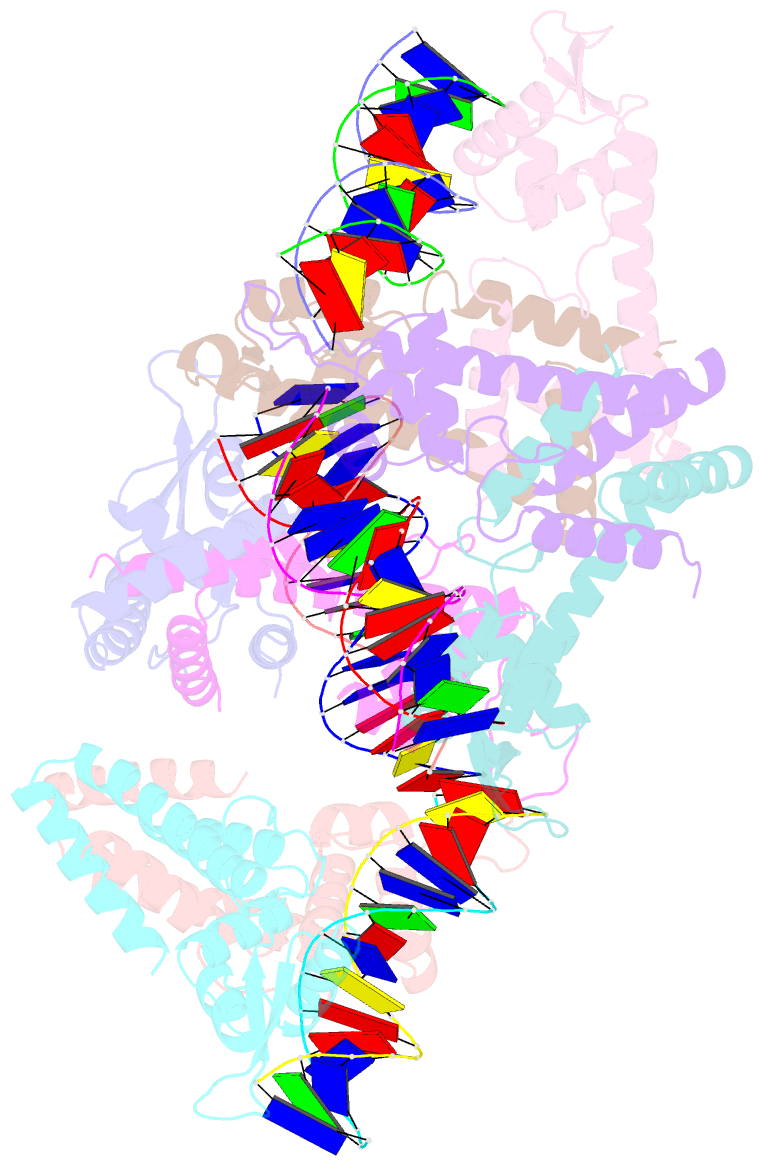
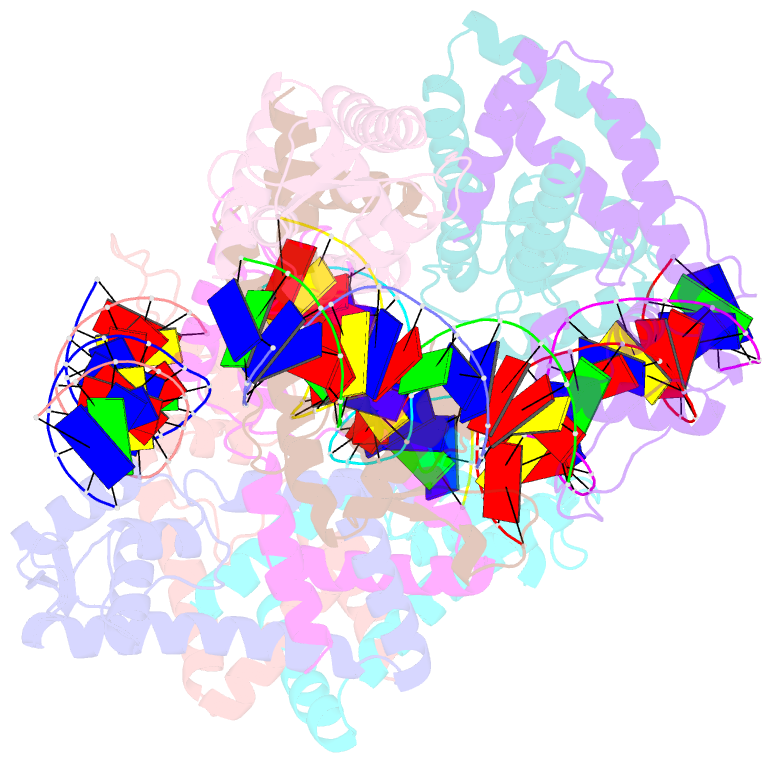
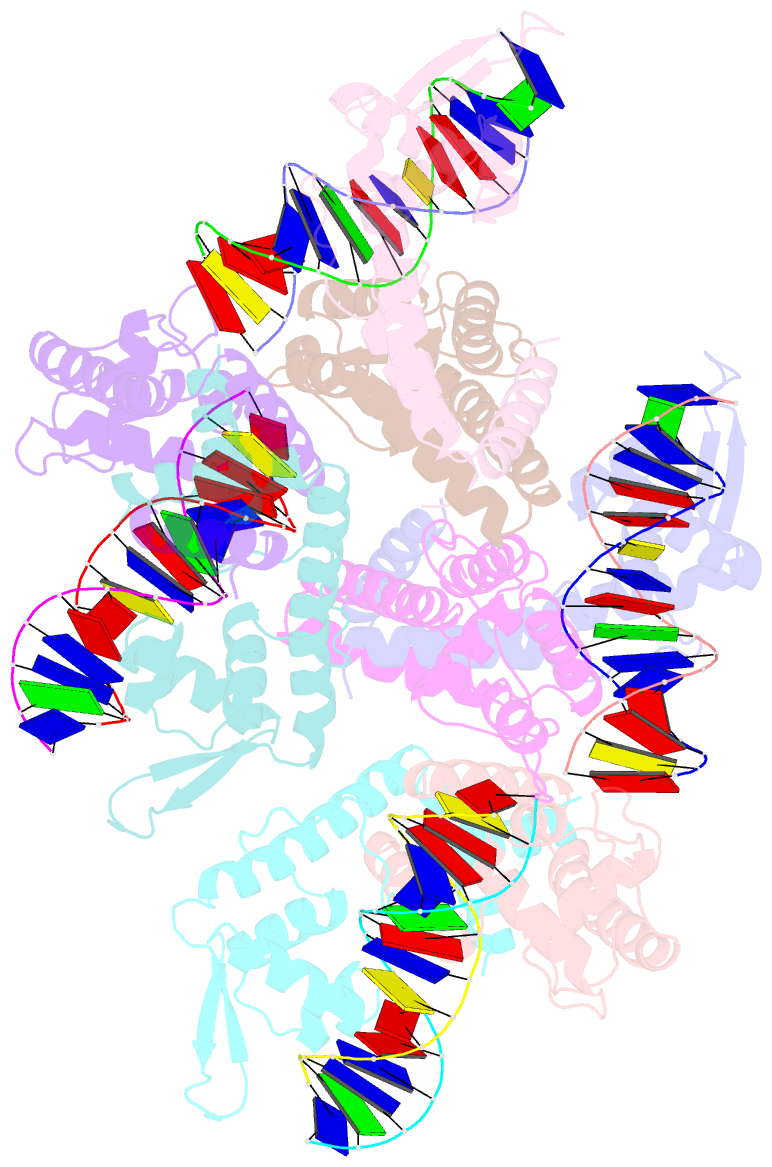
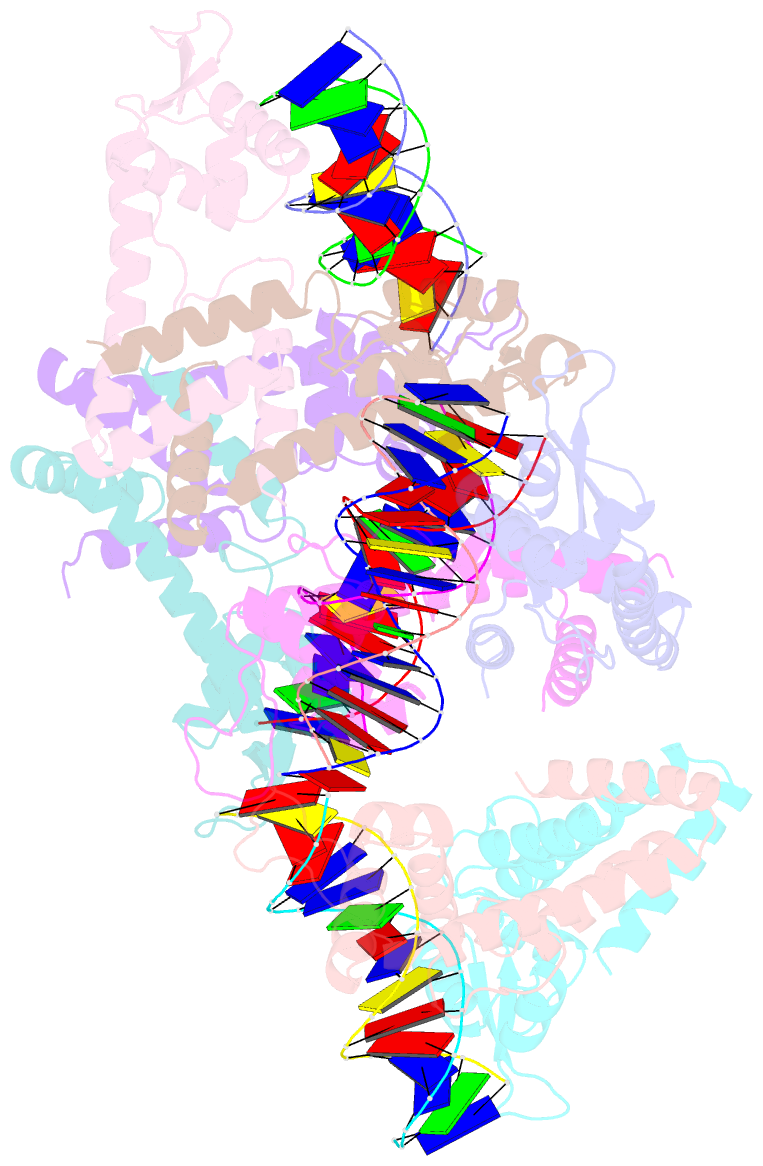
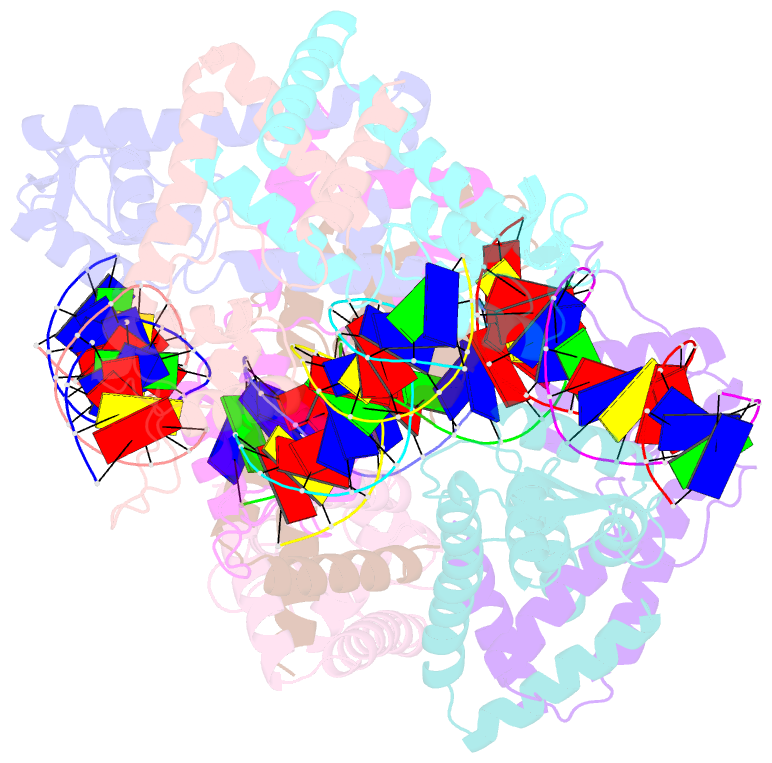
Summary information and primary citation
- PDB-id
- 5yi3; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- metal binding protein-DNA
- Method
- X-ray (2.9 Å)
- Summary
- Structure of lactococcus lactis zitr, c30s mutant in complex with DNA
- Reference
- Zhu R, Song Y, Liu H, Yang Y, Wang S, Yi C, Chen PR (2017): "Allosteric histidine switch for regulation of intracellular zinc(II) fluctuation." Proc.Natl.Acad.Sci.USA, 114, 13661-13666. doi: 10.1073/pnas.1708563115.
- Abstract
- Metalloregulators allosterically control transcriptional activity through metal binding-induced reorganization of ligand residues and/or hydrogen bonding networks, while the coordination atoms on the same ligand residues remain seldom changed. Here we show that the MarR-type zinc transcriptional regulator ZitR switches one of its histidine nitrogen atoms for zinc coordination during the allosteric control of DNA binding. The Zn(II)-coordination nitrogen on histidine 42 within ZitR's high-affinity zinc site (site 1) switches from Nε2 to Nδ1 upon Zn(II) binding to its low-affinity zinc site (site 2), which facilitates ZitR's conversion from the nonoptimal to the optimal DNA-binding conformation. This histidine switch-mediated cooperation between site 1 and site 2 enables ZitR to adjust its DNA-binding affinity in response to a broad range of zinc fluctuation, which may allow the fine tuning of transcriptional regulation.