Summary information and primary citation
- PDB-id
- 5yty; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- antibiotic-DNA
- Method
- X-ray (1.58 Å)
- Summary
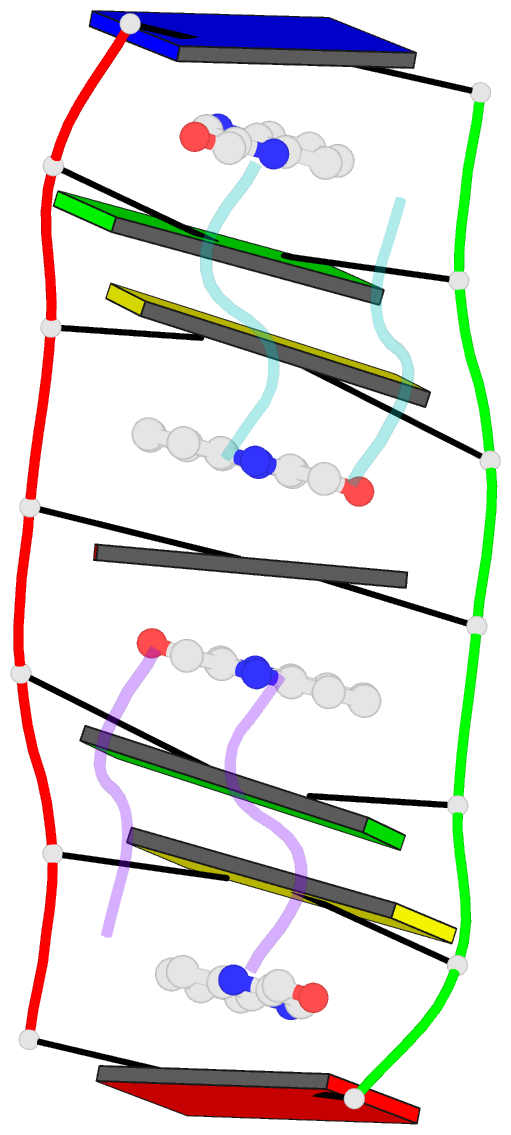
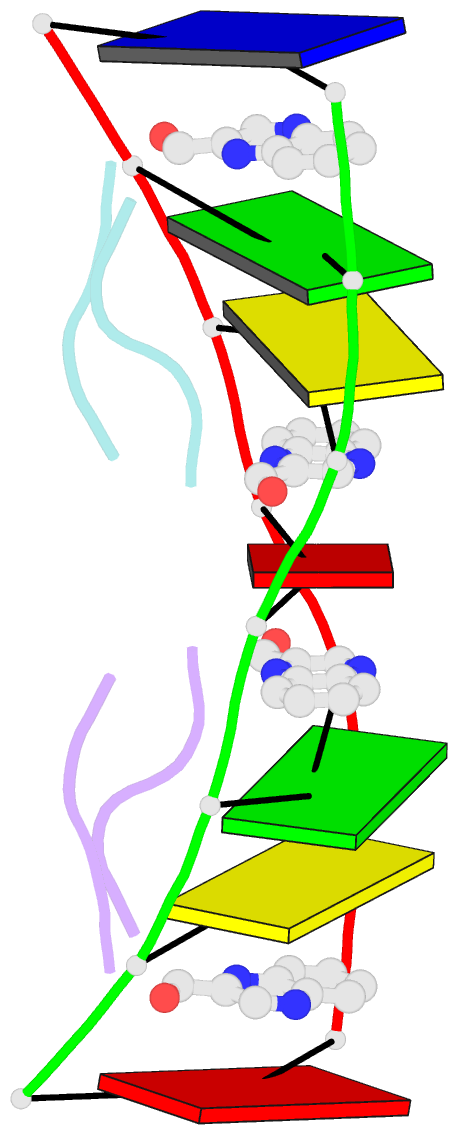
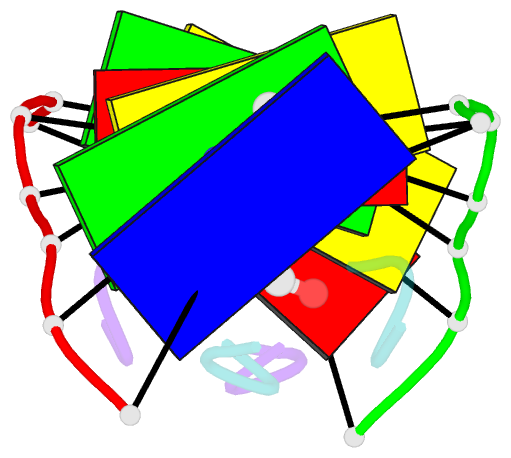
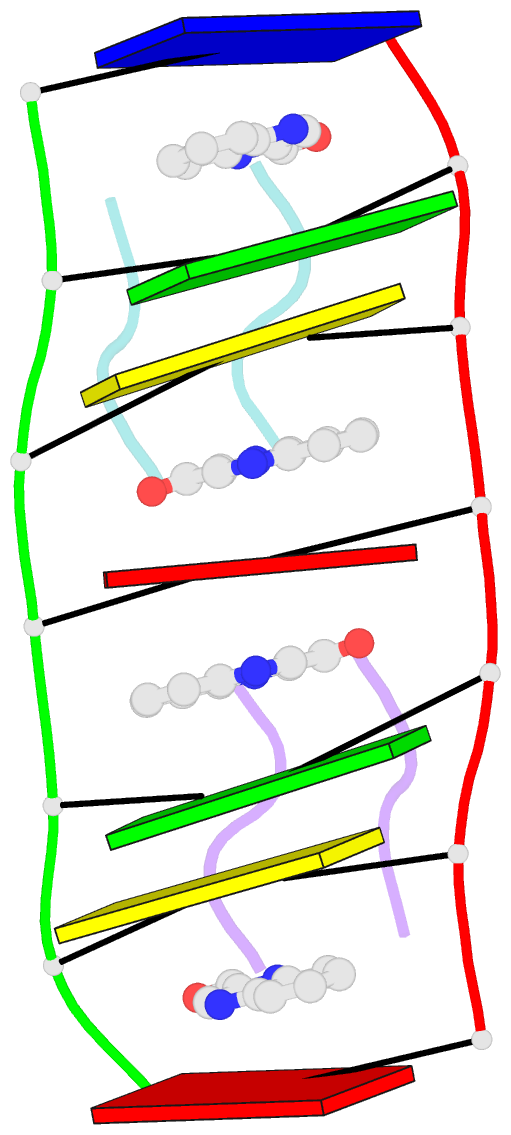
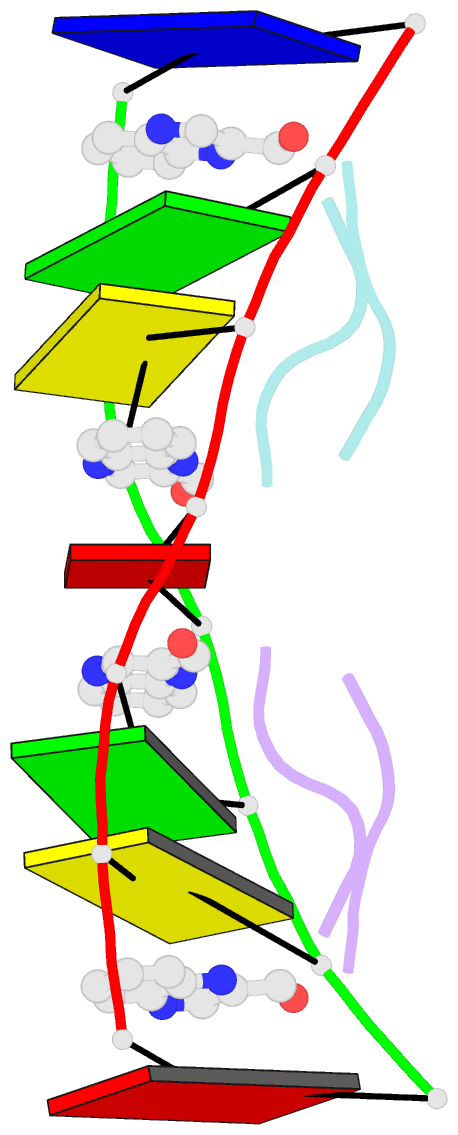
- Crystal structure of echinomycin-d(acgacgt-acgtcgt) complex
- Reference
- Wu PC, Tzeng SL, Chang CK, Kao YF, Waring MJ, Hou MH (2018): "Cooperative recognition of T:T mismatch by echinomycin causes structural distortions in DNA duplex." Nucleic Acids Res., 46, 7396-7404. doi: 10.1093/nar/gky345.
- Abstract
- Small-molecule compounds that target mismatched base pairs in DNA offer a novel prospective for cancer diagnosis and therapy. The potent anticancer antibiotic echinomycin functions by intercalating into DNA at CpG sites. Surprisingly, we found that the drug strongly prefers to bind to consecutive CpG steps separated by a single T:T mismatch. The preference appears to result from enhanced cooperativity associated with the binding of the second echinomycin molecule. Crystallographic studies reveal that this preference originates from the staggered quinoxaline rings of the two neighboring antibiotic molecules that surround the T:T mismatch forming continuous stacking interactions within the duplex. These and other associated changes in DNA conformation allow the formation of a minor groove pocket for tight binding of the second echinomycin molecule. We also show that echinomycin displays enhanced cytotoxicity against mismatch repair-deficient cell lines, raising the possibility of repurposing the drug for detection and treatment of mismatch repair-deficient cancers.