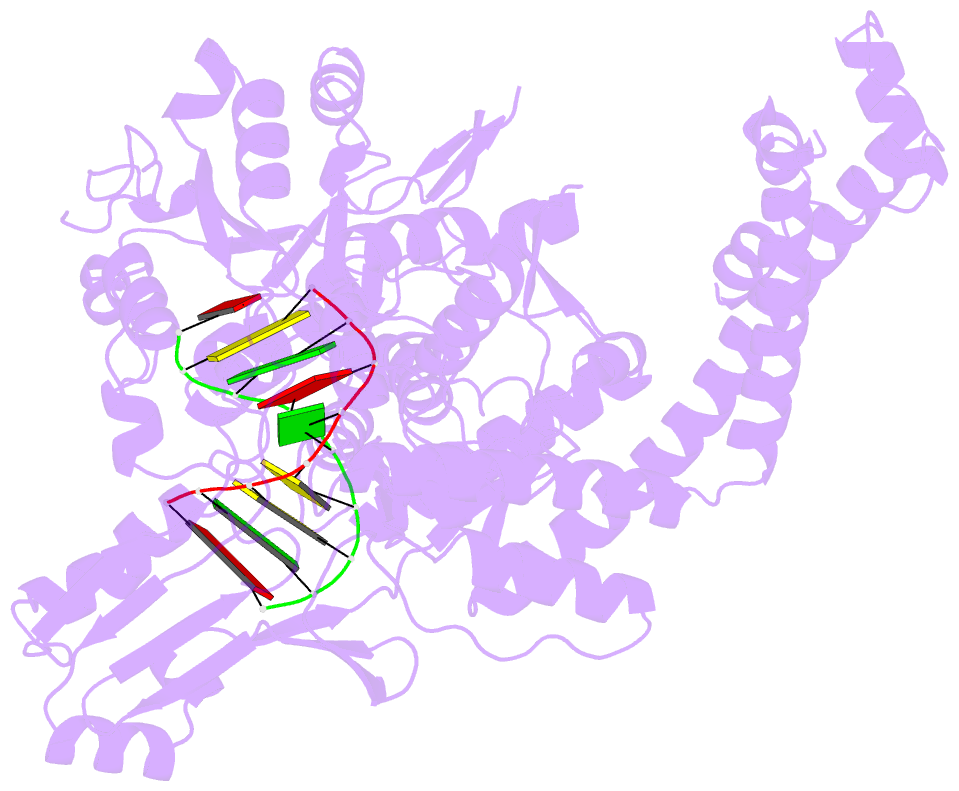
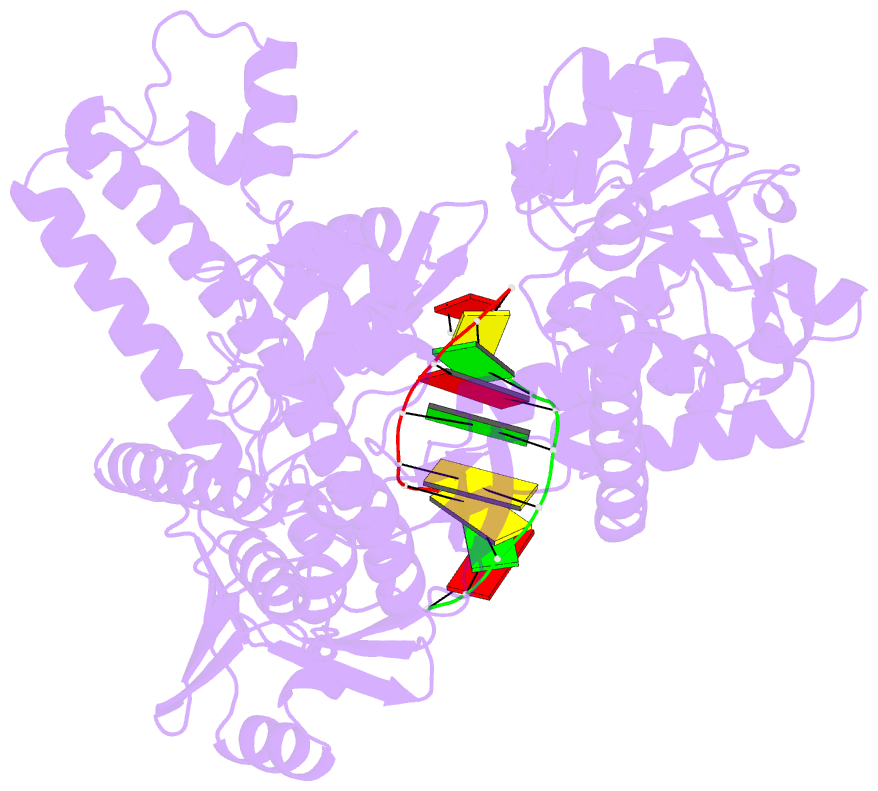
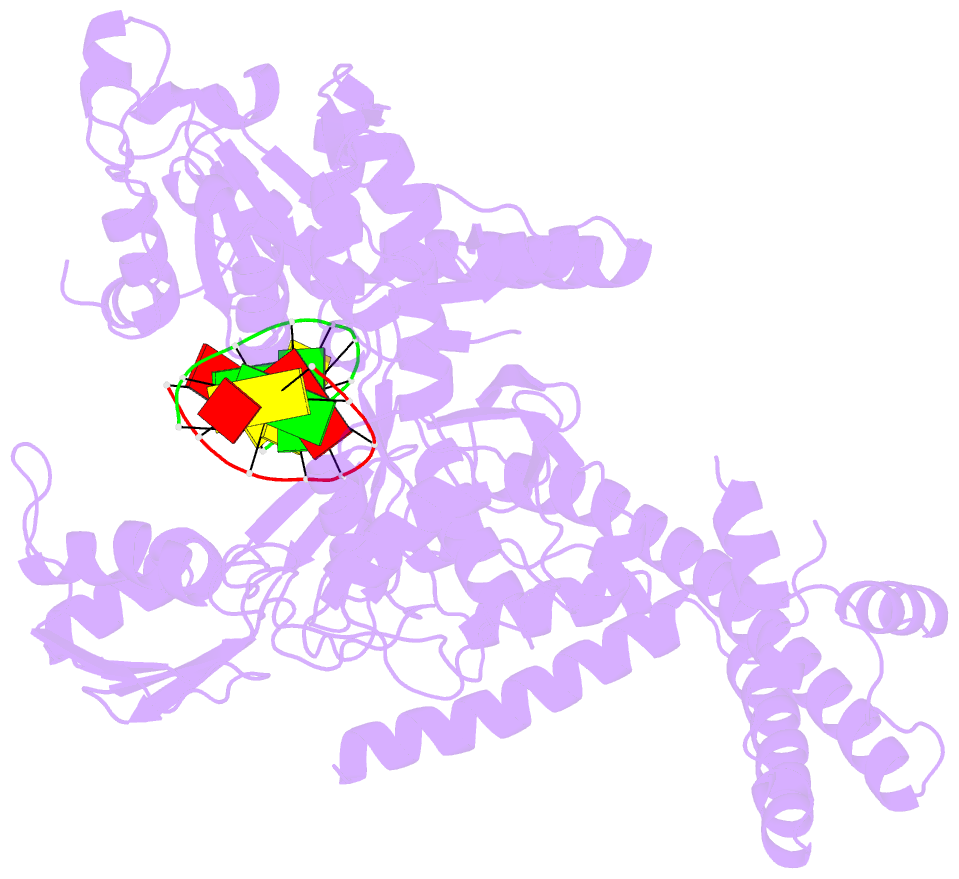
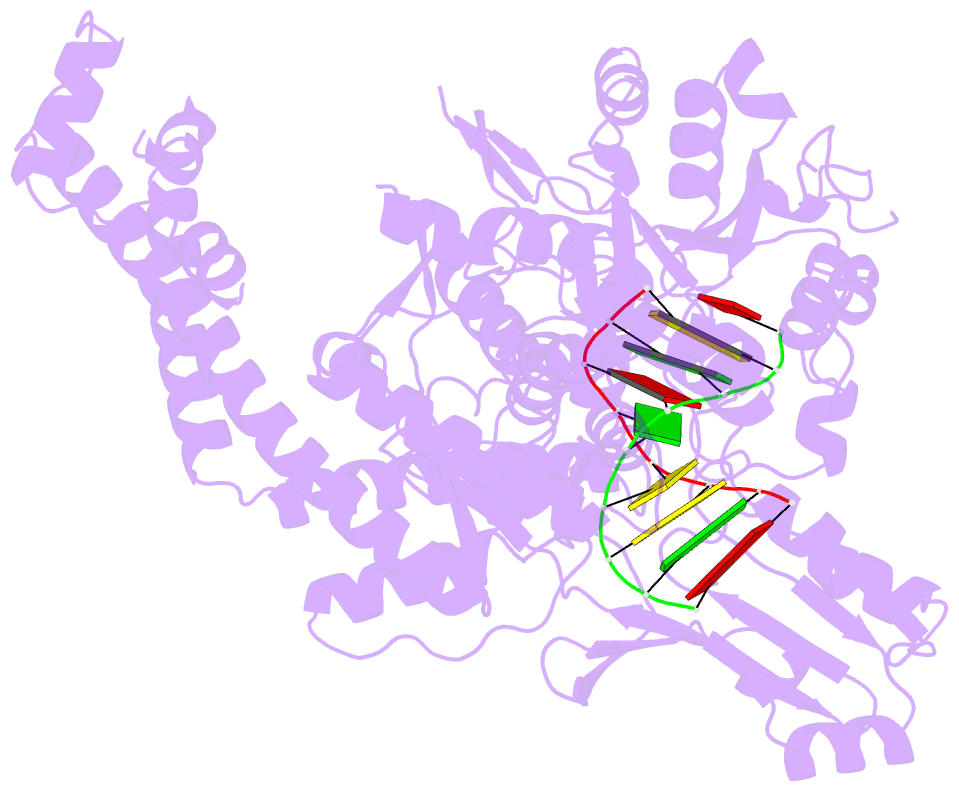
Summary information and primary citation
- PDB-id
- 5zen; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- isomerase-DNA
- Method
- X-ray (2.75 Å)
- Summary
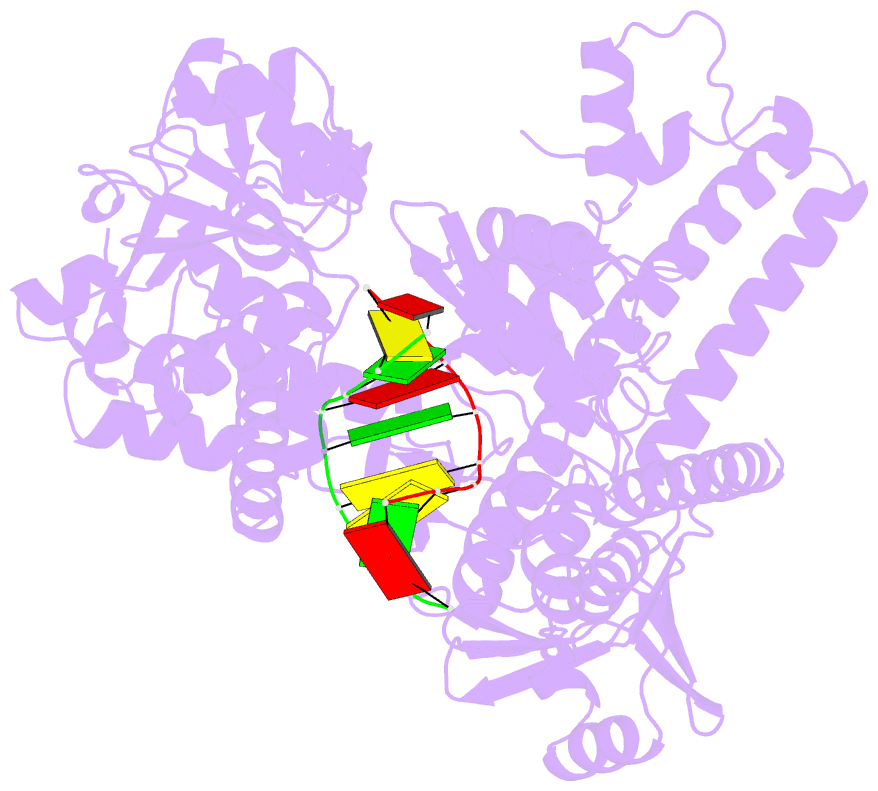
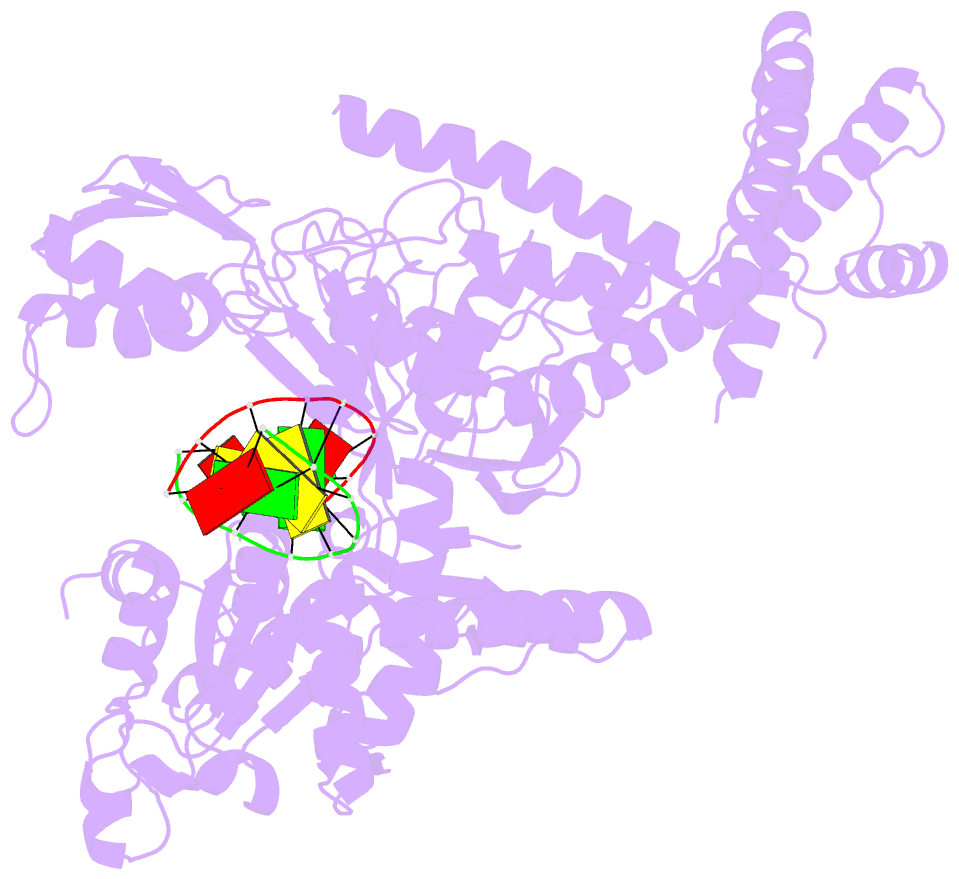
- Crystal structure of human topoisomerase ii beta in complex with DNA: a new quaternary conformation showing opening of the protein-linked DNA-gate
- Reference
- Chen SF, Huang NL, Lin JH, Wu CC, Wang YR, Yu YJ, Gilson MK, Chan NL (2018): "Structural insights into the gating of DNA passage by the topoisomerase II DNA-gate." Nat Commun, 9, 3085. doi: 10.1038/s41467-018-05406-y.
- Abstract
- Type IIA topoisomerases (Top2s) manipulate the handedness of DNA crossovers by introducing a transient and protein-linked double-strand break in one DNA duplex, termed the DNA-gate, whose opening allows another DNA segment to be transported through to change the DNA topology. Despite the central importance of this gate-opening event to Top2 function, the DNA-gate in all reported structures of Top2-DNA complexes is in the closed state. Here we present the crystal structure of a human Top2 DNA-gate in an open conformation, which not only reveals structural characteristics of its DNA-conducting path, but also uncovers unexpected yet functionally significant conformational changes associated with gate-opening. This structure further implicates Top2's preference for a left-handed DNA braid and allows the construction of a model representing the initial entry of another DNA duplex into the DNA-gate. Steered molecular dynamics calculations suggests the Top2-catalyzed DNA passage may be achieved by a rocker-switch-type movement of the DNA-gate.