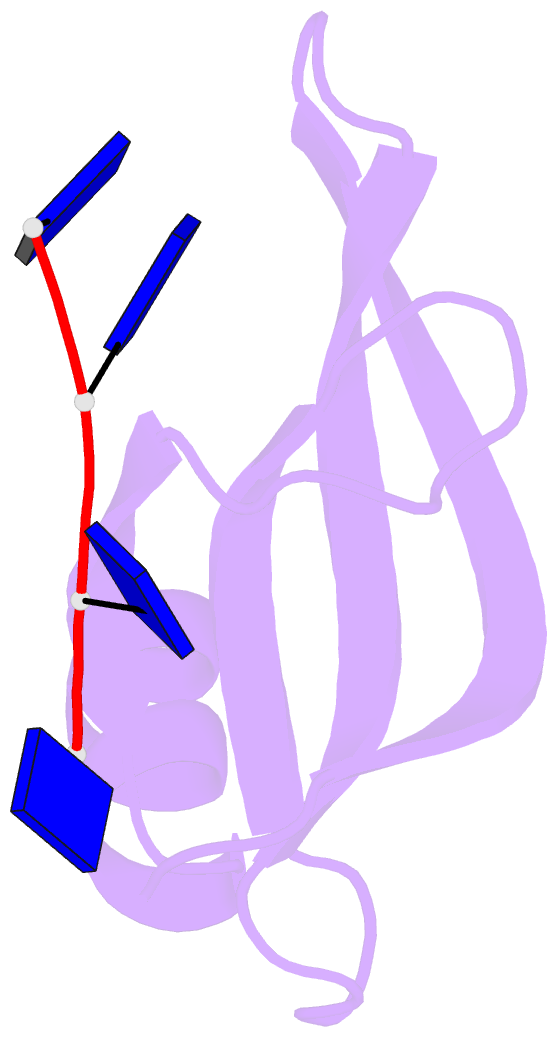
Summary information and primary citation
- PDB-id
- 5zkl; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- unknown function-DNA
- Method
- X-ray (1.951 Å)
- Summary
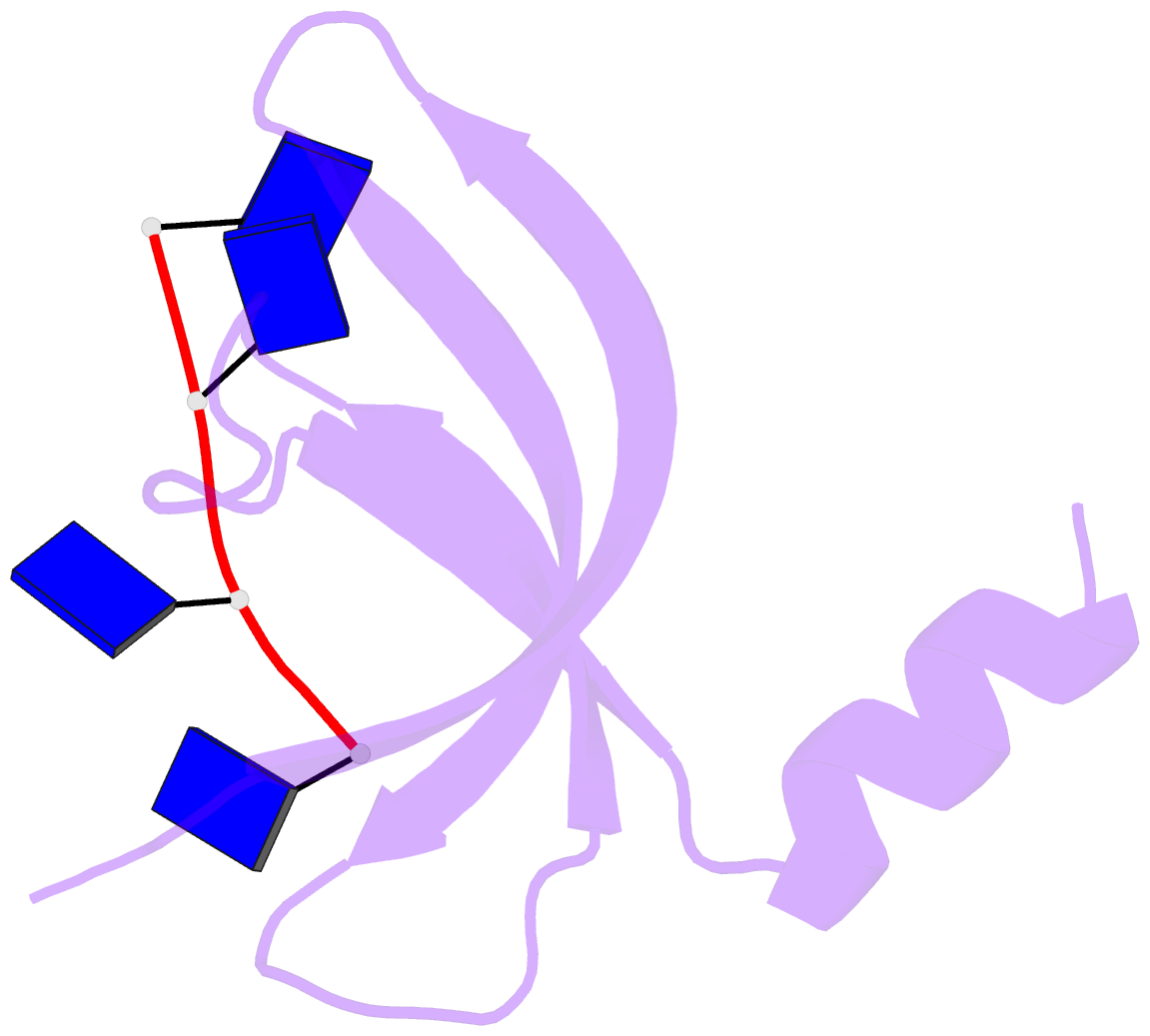
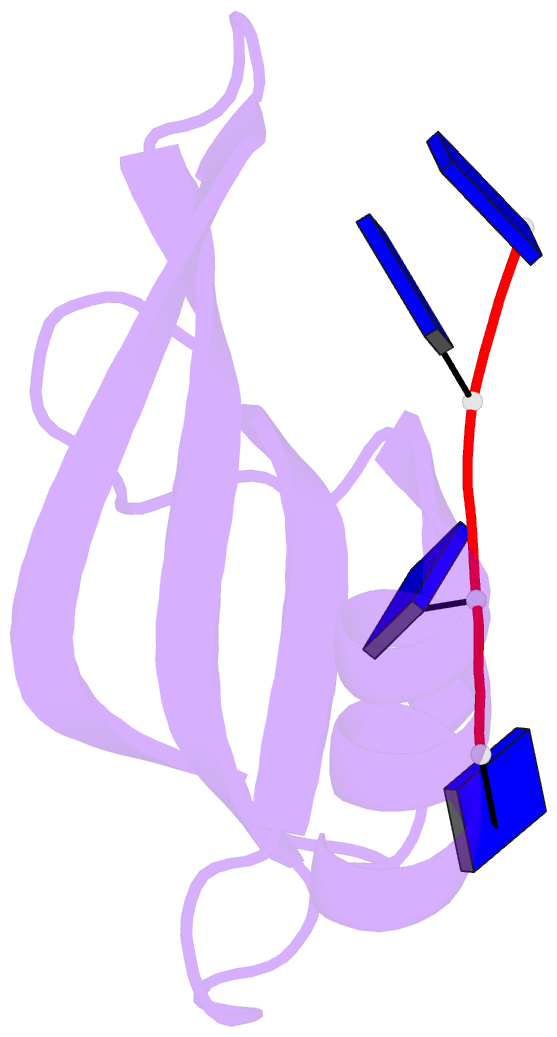
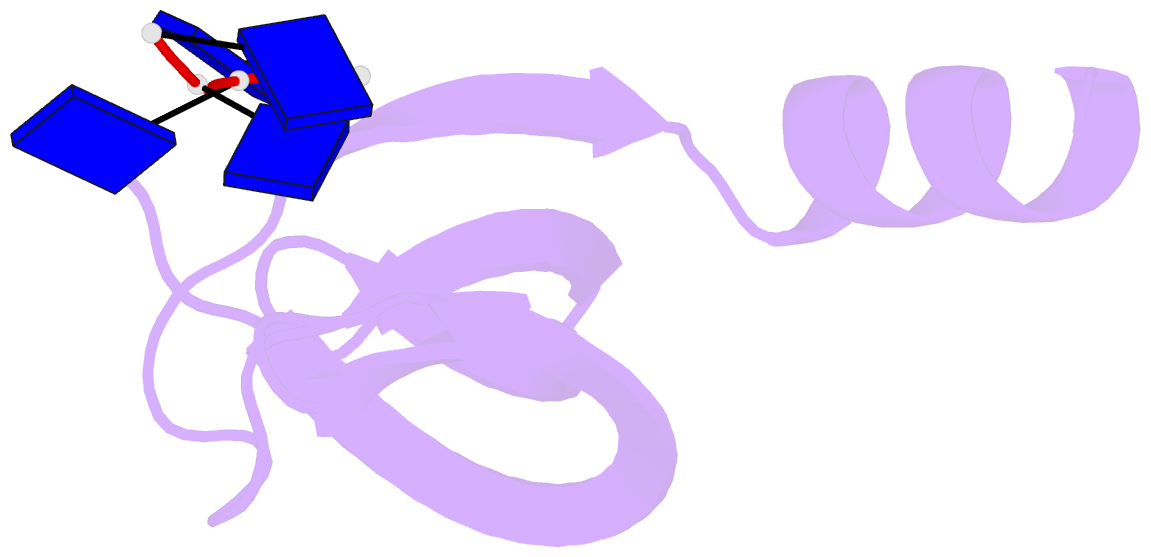
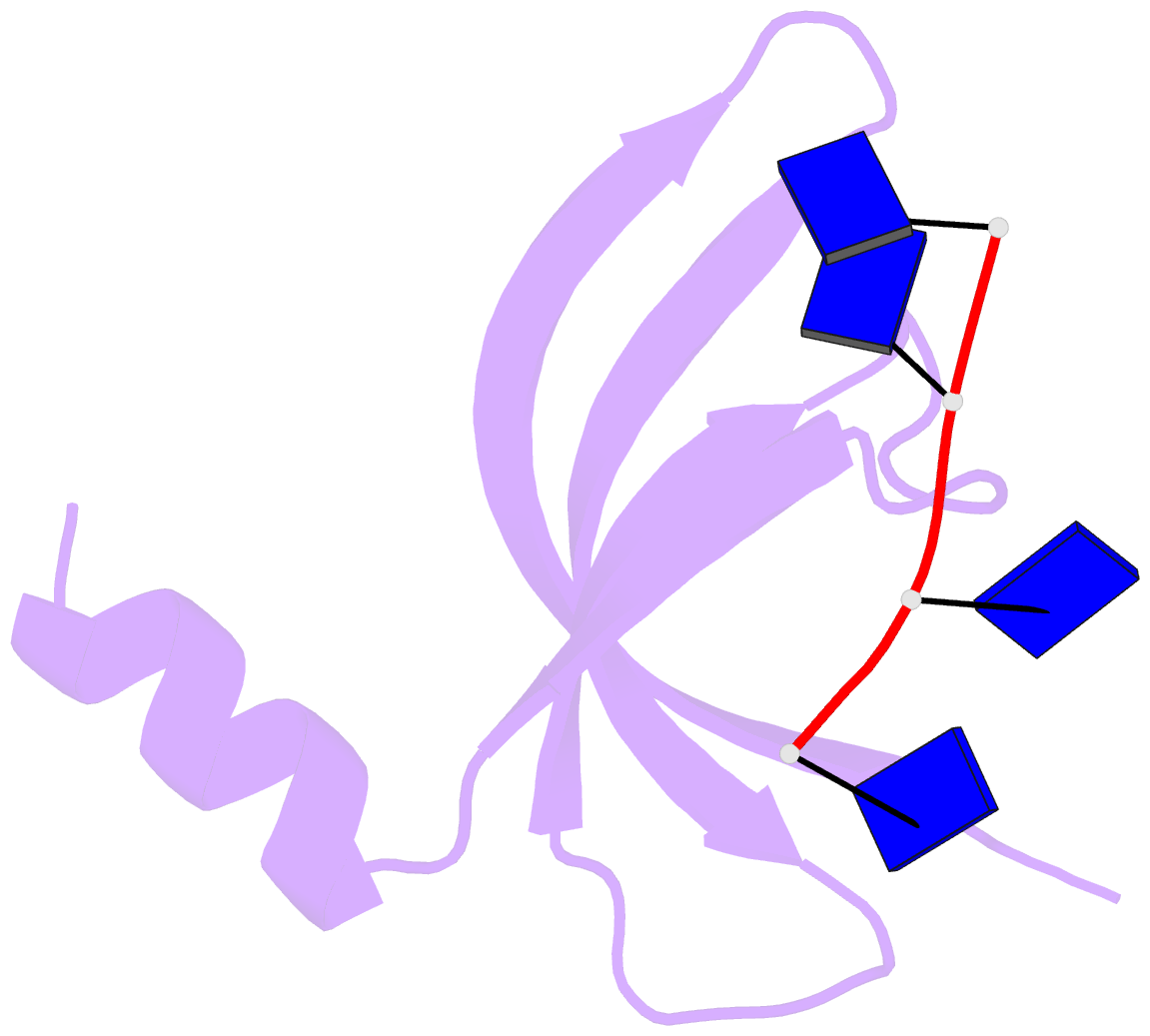
- Crystal structure of streptococcus pneumoniae sp_0782 (residues 7-79) in complex with single-stranded DNA dt12
- Reference
- Li S, Lu G, Fang X, Ramelot TA, Kennedy MA, Zhou X, Gong P, Zhang X, Liu M, Zhu J, Yang Y (2020): "Structural insight into the length-dependent binding of ssDNA by SP_0782 from Streptococcus pneumoniae, reveals a divergence in the DNA-binding interface of PC4-like proteins." Nucleic Acids Res., 48, 432-444. doi: 10.1093/nar/gkz1045.
- Abstract
- SP_0782 from Streptococcus pneumoniae is a dimeric protein that potentially binds with single-stranded DNA (ssDNA) in a manner similar to human PC4, the prototype of PC4-like proteins, which plays roles in transcription and maintenance of genome stability. In a previous NMR study, SP_0782 exhibited an ssDNA-binding property different from YdbC, a prokaryotic PC4-like protein from Lactococcus lactis, but the underlying mechanism remains unclear. Here, we show that although SP_0782 adopts an overall fold similar to those of PC4 and YdbC, the ssDNA length occupied by SP_0782 is shorter than those occupied by PC4 and YdbC. SP_0782 exhibits varied binding patterns for different lengths of ssDNA, and tends to form large complexes with ssDNA in a potential high-density binding manner. The structures of SP_0782 complexed with different ssDNAs reveal that the varied binding patterns are associated with distinct capture of nucleotides in two major DNA-binding regions of SP_0782. Moreover, a comparison of known structures of PC4-like proteins complexed with ssDNA reveals a divergence in the binding interface between prokaryotic and eukaryotic PC4-like proteins. This study provides insights into the ssDNA-binding mechanism of PC4-like proteins, and benefits further study regarding the biological function of SP_0782, probably in DNA protection and natural transformation.