Summary information and primary citation
- PDB-id
- 5zux; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-DNA
- Method
- NMR
- Summary
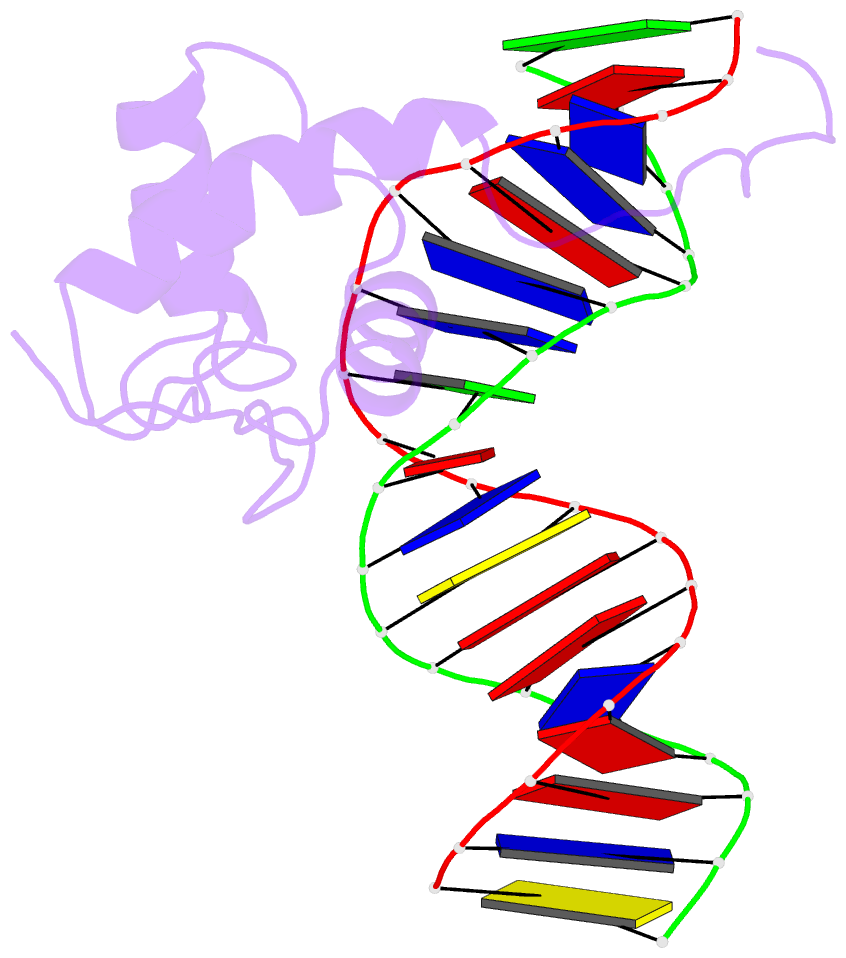
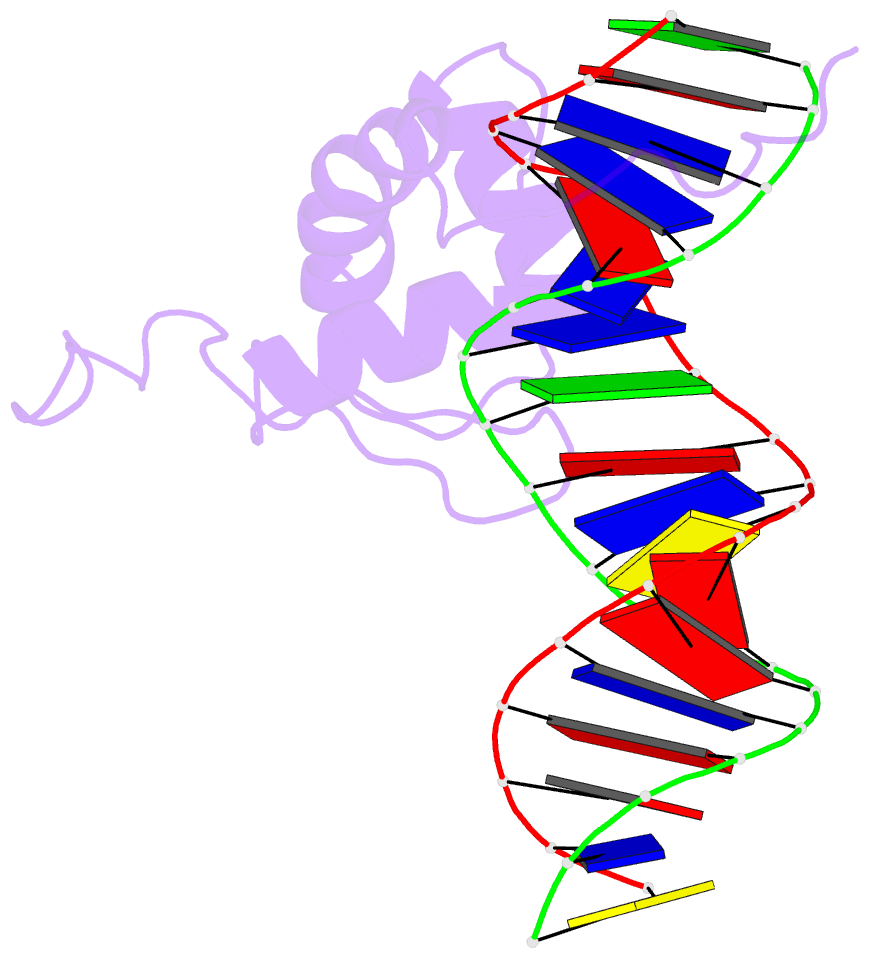
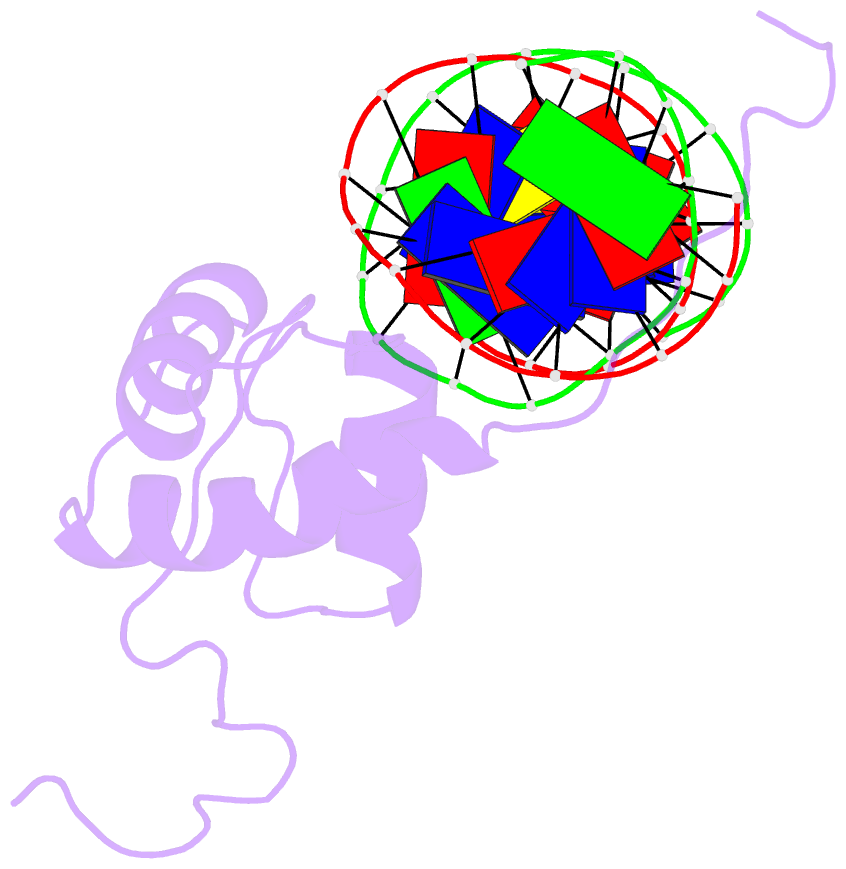
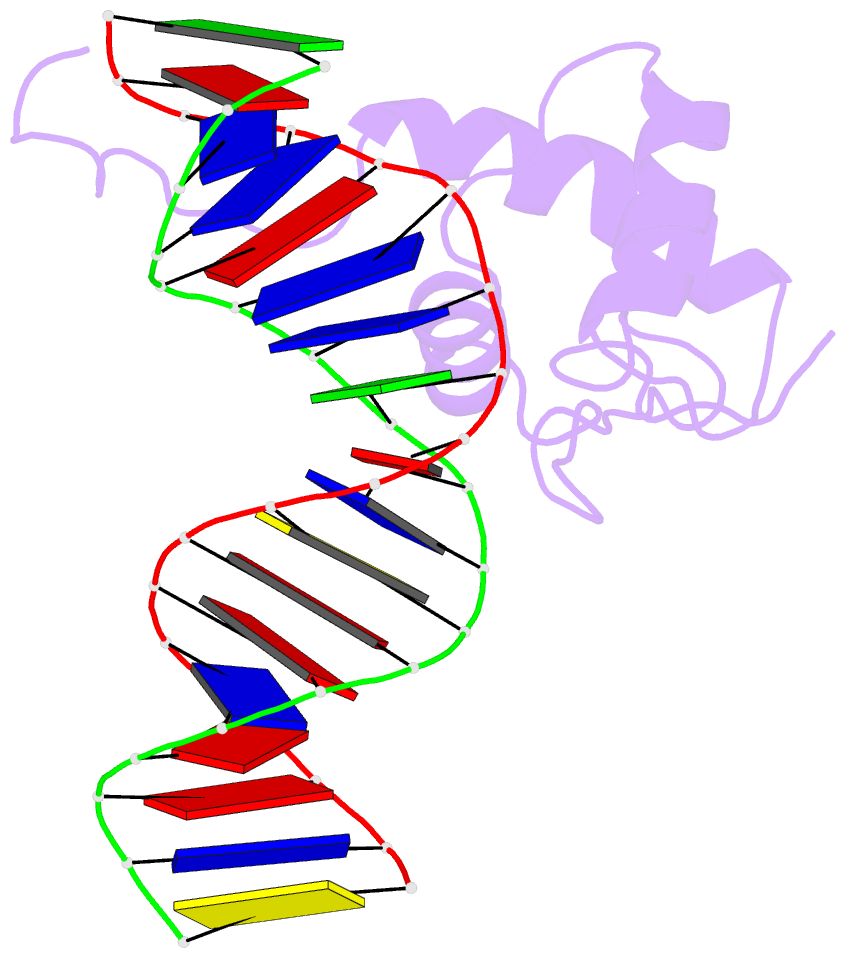
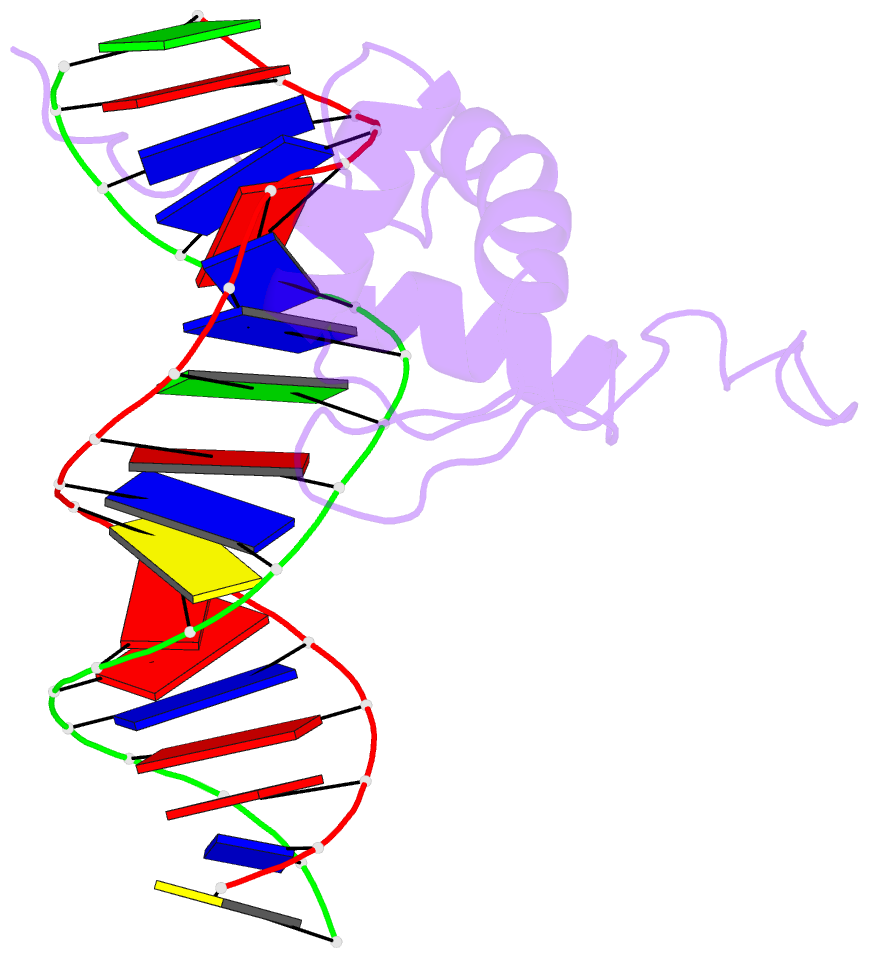
- Solution structure of the DNA complex of the c-terminal domain of rok
- Reference
- Duan B, Ding P, Hughes TR, Navarre WW, Liu J, Xia B (2018): "How bacterial xenogeneic silencer rok distinguishes foreign from self DNA in its resident genome." Nucleic Acids Res., 46, 10514-10529. doi: 10.1093/nar/gky836.
- Abstract
- Bacterial xenogeneic silencers play important roles in bacterial evolution by recognizing and inhibiting expression from foreign genes acquired through horizontal gene transfer, thereby buffering against potential fitness consequences of their misregulated expression. Here, the detailed DNA binding properties of Rok, a xenogeneic silencer in Bacillus subtilis, was studied using protein binding microarray, and the solution structure of its C-terminal DNA binding domain was determined in complex with DNA. The C-terminal domain of Rok adopts a typical winged helix fold, with a novel DNA recognition mechanism different from other winged helix proteins or xenogeneic silencers. Rok binds the DNA minor groove by forming hydrogen bonds to bases through N154, T156 at the N-terminal of α3 helix and R174 of wing W1, assisted by four lysine residues interacting electrostatically with DNA backbone phosphate groups. These structural features endow Rok with preference towards DNA sequences harboring AACTA, TACTA, and flexible multiple TpA steps, while rigid A-tracts are disfavored. Correspondingly, the Bacillus genomes containing Rok are rich in A-tracts and show a dramatic underrepresentation of AACTA and TACTA, which are significantly enriched in Rok binding regions. These observations suggest that the xenogeneic silencing protein and its resident genome may have evolved cooperatively.