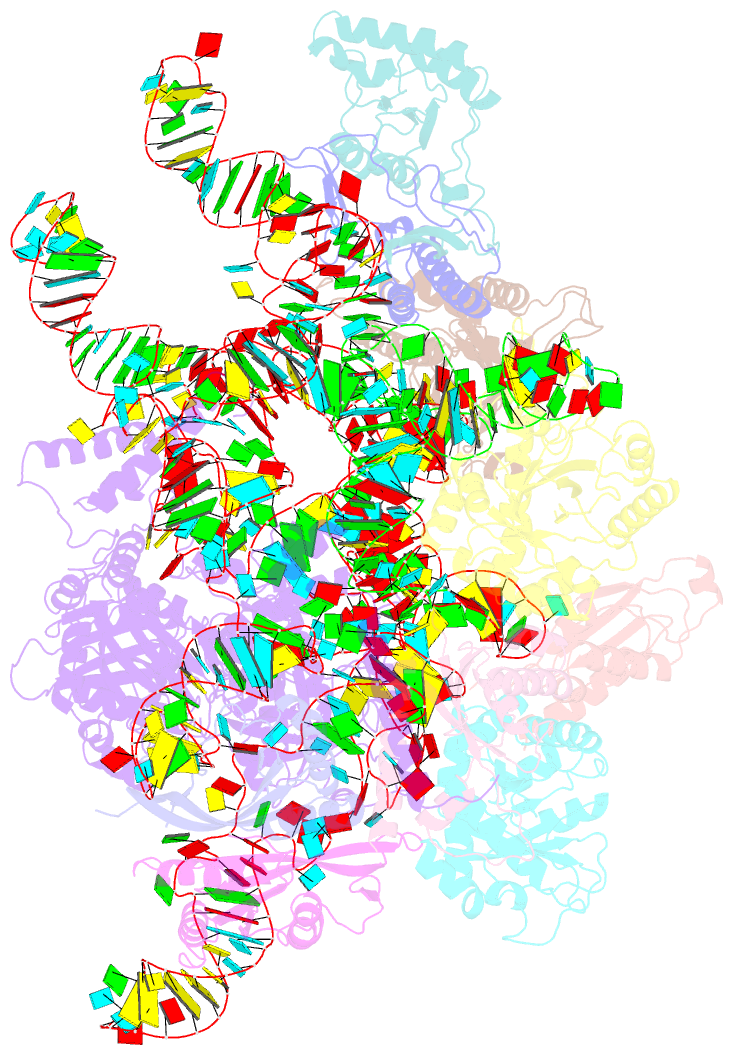
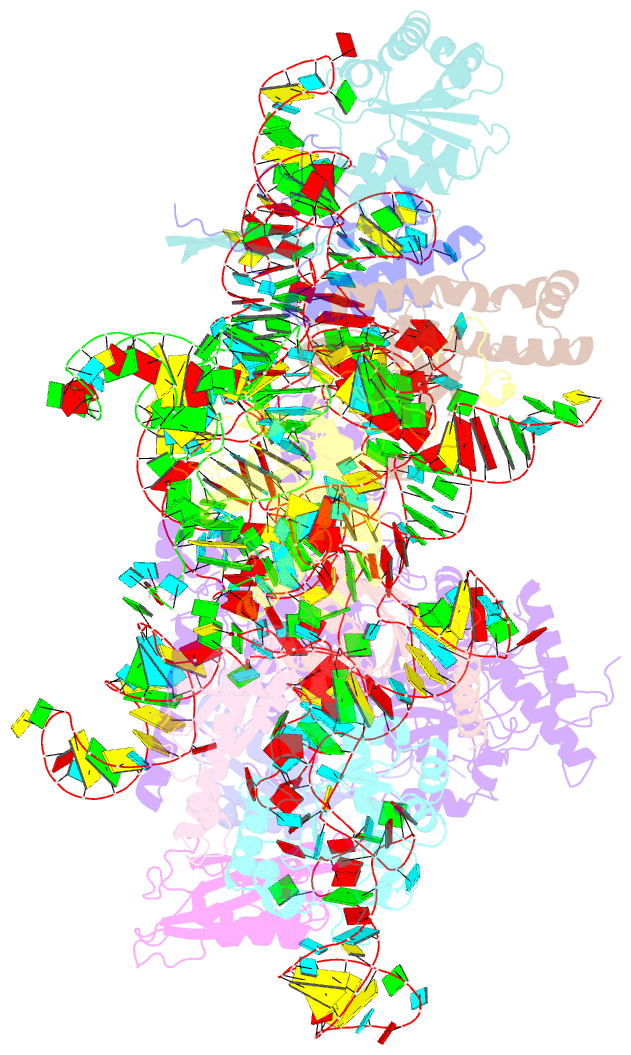
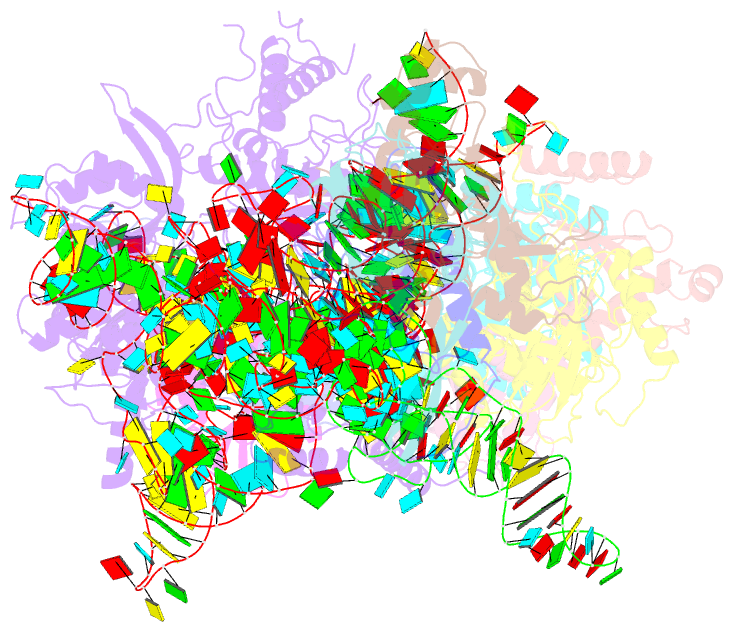
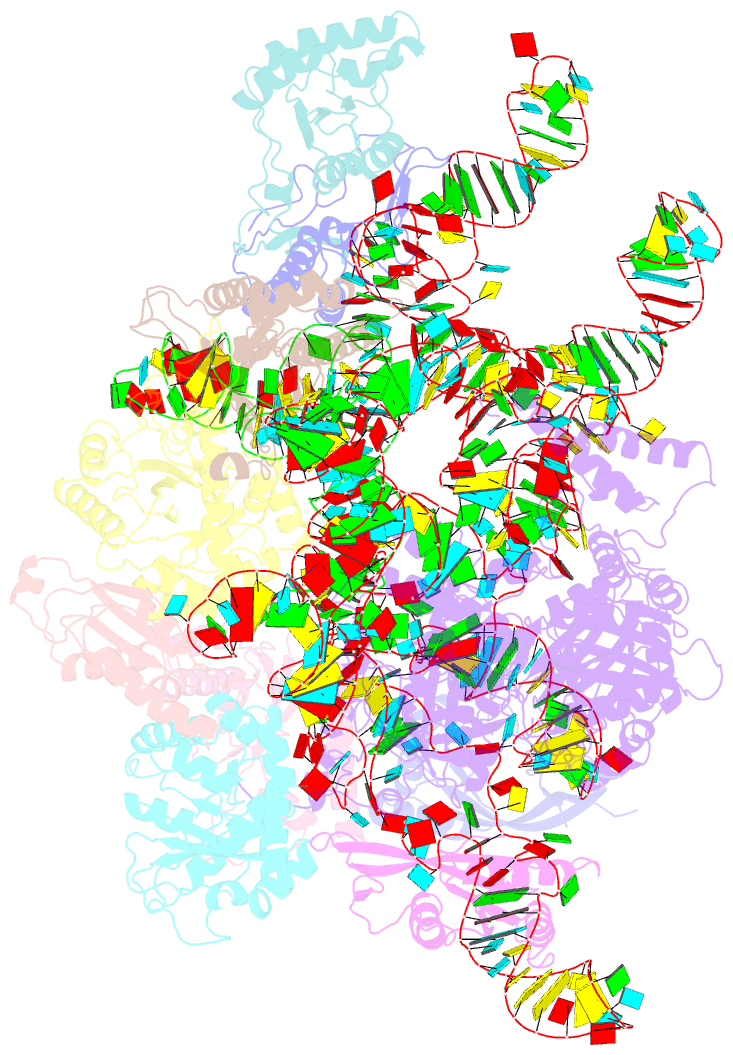
Summary information and primary citation
- PDB-id
- 6ah3; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-RNA
- Method
- cryo-EM (3.48 Å)
- Summary
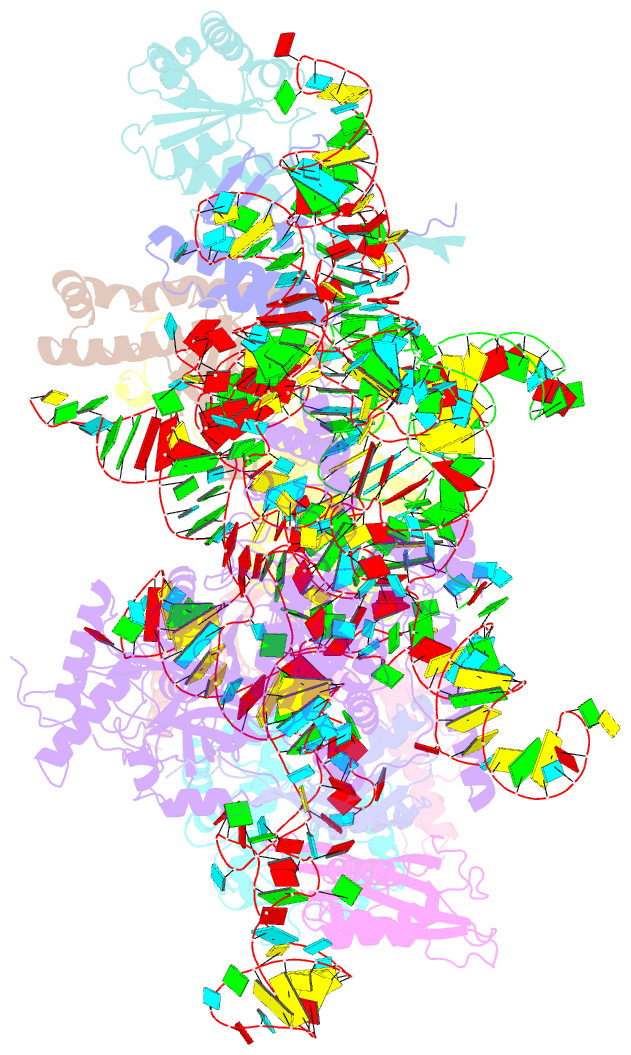
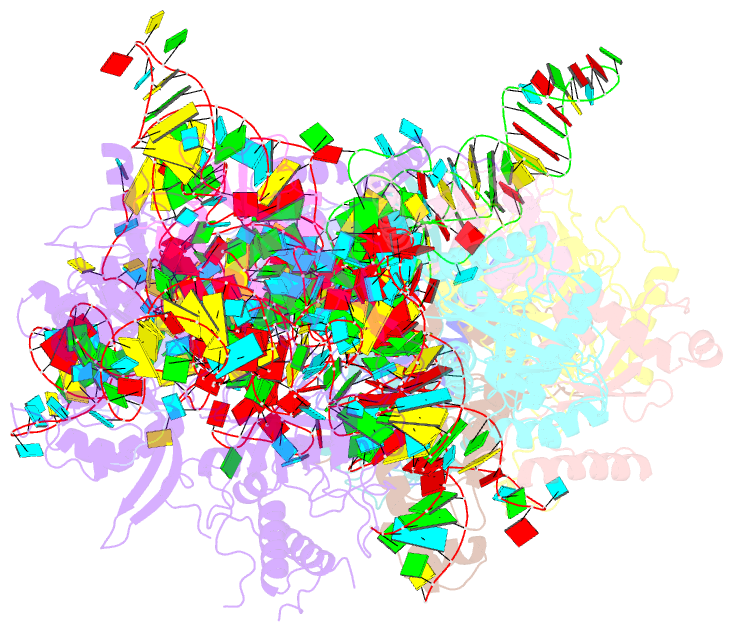
- cryo-EM structure of yeast ribonuclease p with pre-trna substrate
- Reference
- Lan P, Tan M, Zhang Y, Niu S, Chen J, Shi S, Qiu S, Wang X, Peng X, Cai G, Cheng H, Wu J, Li G, Lei M (2018): "Structural insight into precursor tRNA processing by yeast ribonuclease P." Science, 362. doi: 10.1126/science.aat6678.
- Abstract
- Ribonuclease P (RNase P) is a universal ribozyme responsible for processing the 5'-leader of pre-transfer RNA (pre-tRNA). Here, we report the 3.5-angstrom cryo-electron microscopy structures of Saccharomyces cerevisiae RNase P alone and in complex with pre-tRNAPhe The protein components form a hook-shaped architecture that wraps around the RNA and stabilizes RNase P into a "measuring device" with two fixed anchors that recognize the L-shaped pre-tRNA. A universally conserved uridine nucleobase and phosphate backbone in the catalytic center together with the scissile phosphate and the O3' leaving group of pre-tRNA jointly coordinate two catalytic magnesium ions. Binding of pre-tRNA induces a conformational change in the catalytic center that is required for catalysis. Moreover, simulation analysis suggests a two-metal-ion SN2 reaction pathway of pre-tRNA cleavage. These results not only reveal the architecture of yeast RNase P but also provide a molecular basis of how the 5'-leader of pre-tRNA is processed by eukaryotic RNase P.