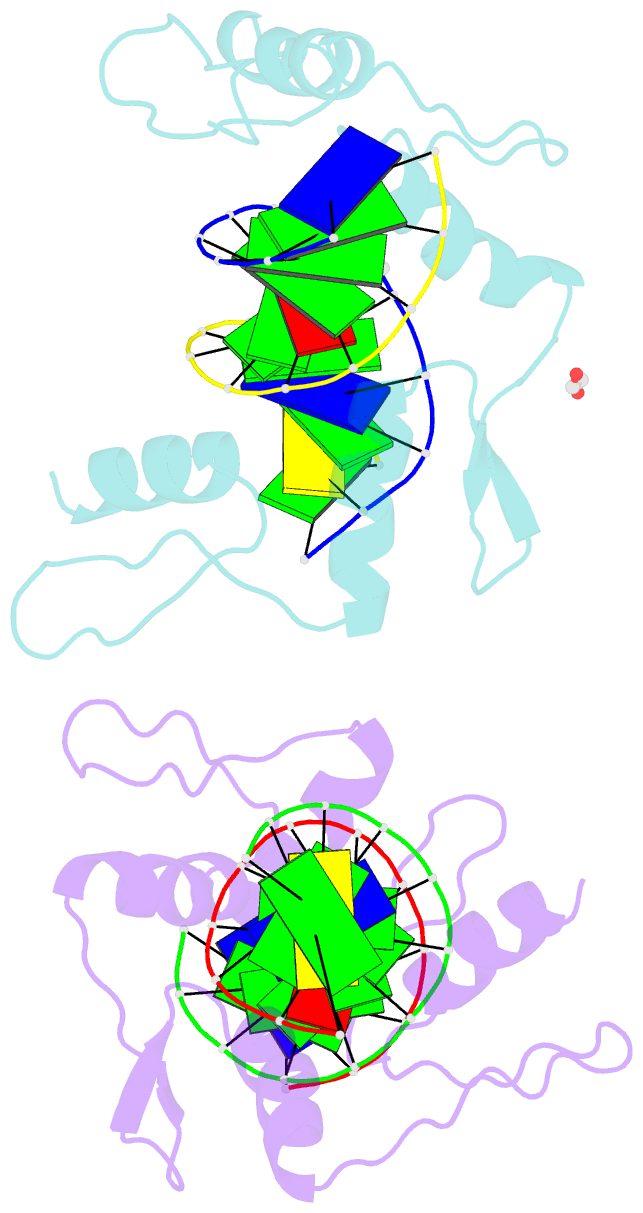
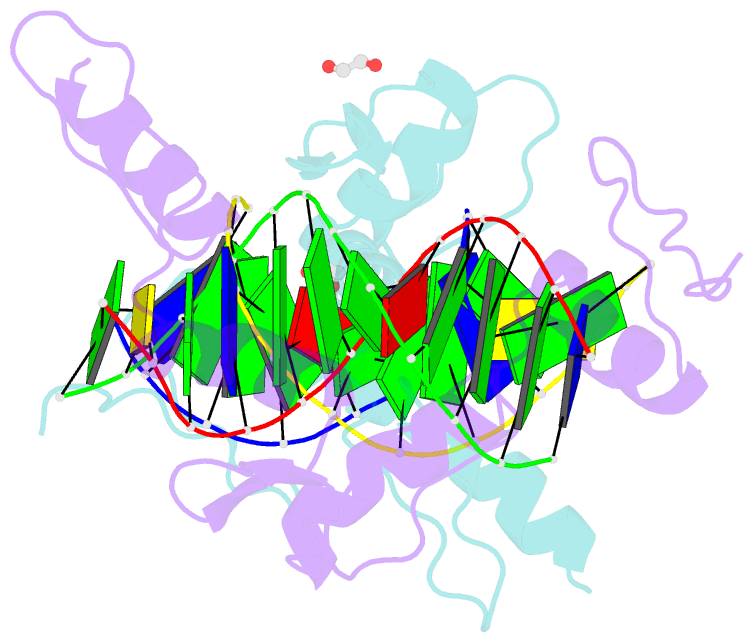
Summary information and primary citation
- PDB-id
- 6b0p; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (2.077 Å)
- Summary
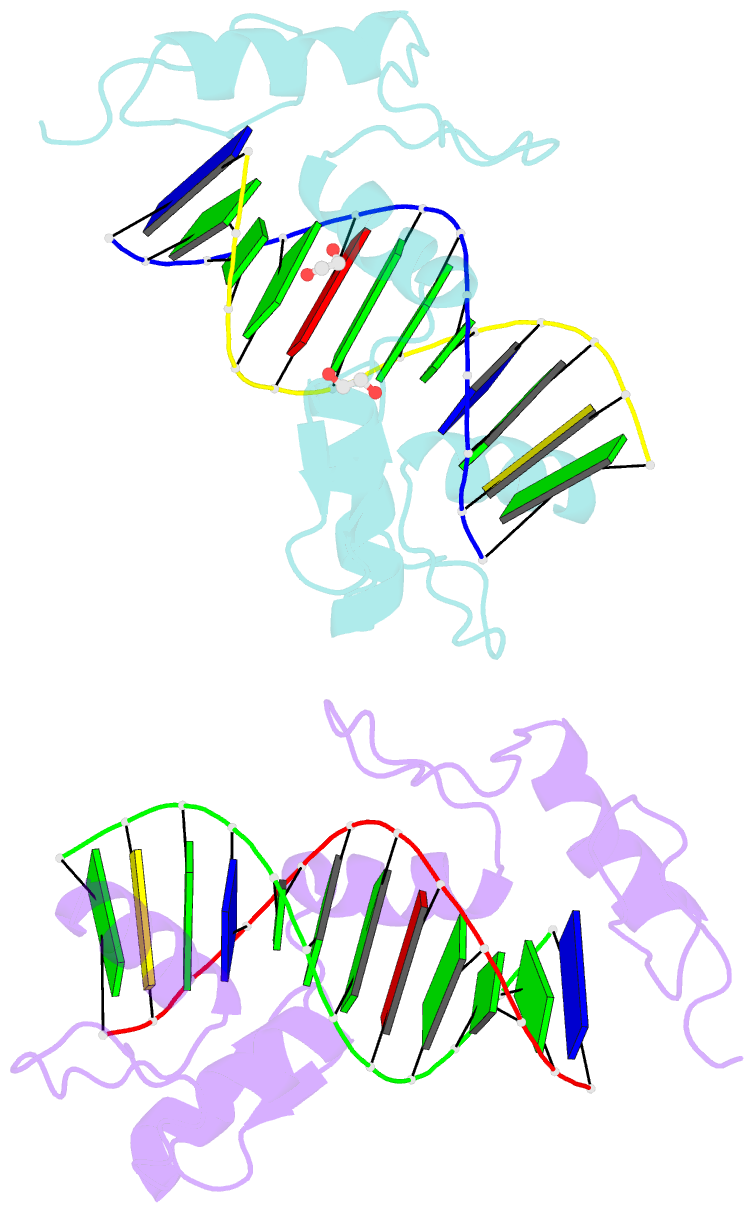
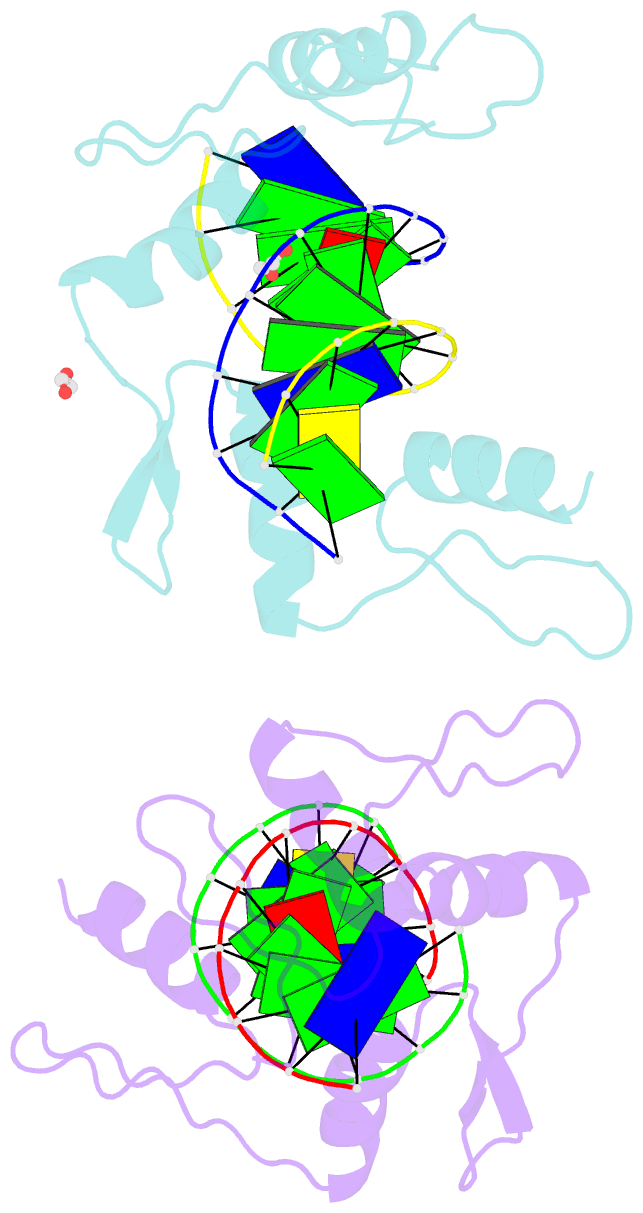
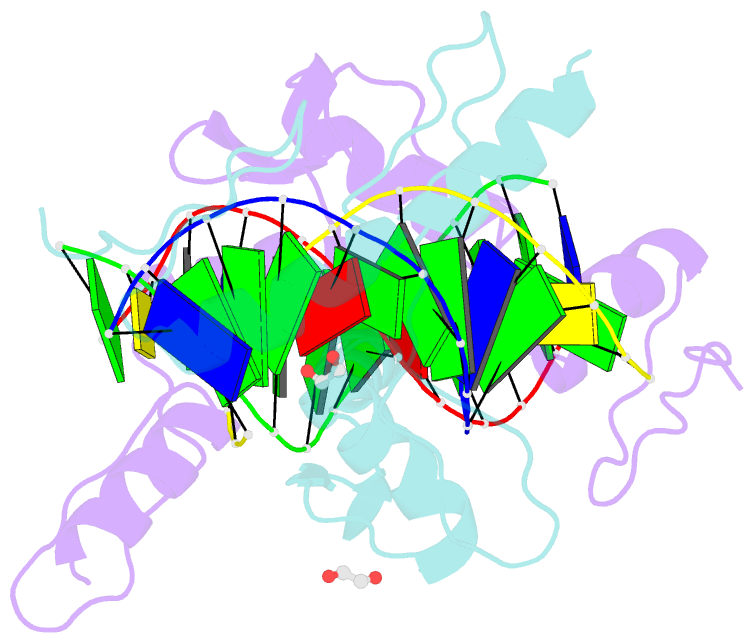
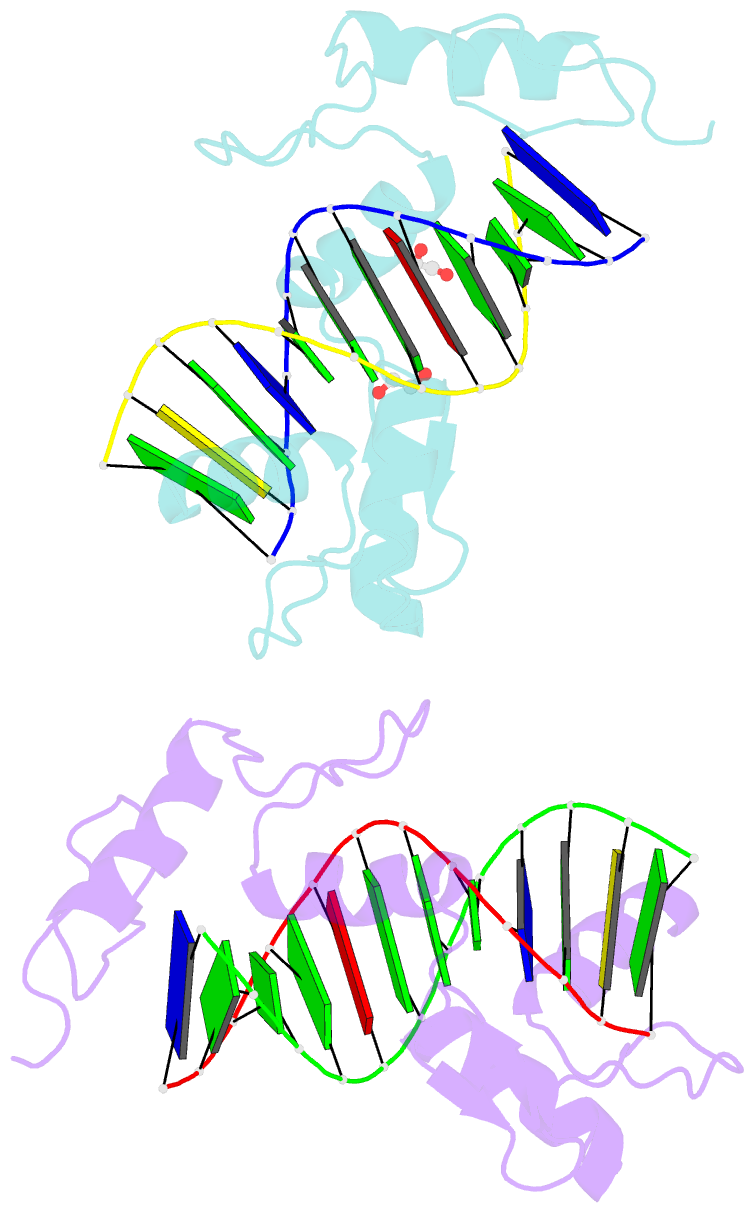
- Zinc finger domain of wt1(-kts form) with 12+1mer oligonucleotide with 3' triplet ggt
- Reference
- Wang D, Horton JR, Zheng Y, Blumenthal RM, Zhang X, Cheng X (2018): "Role for first zinc finger of WT1 in DNA sequence specificity: Denys-Drash syndrome-associated WT1 mutant in ZF1 enhances affinity for a subset of WT1 binding sites." Nucleic Acids Res., 46, 3864-3877. doi: 10.1093/nar/gkx1274.
- Abstract
- Wilms tumor protein (WT1) is a Cys2-His2 zinc-finger transcription factor vital for embryonic development of the genitourinary system. The protein contains a C-terminal DNA binding domain with four tandem zinc-fingers (ZF1-4). An alternative splicing of Wt1 can add three additional amino acids-lysine (K), threonine (T) and serine (S)-between ZF3 and ZF4. In the -KTS isoform, ZF2-4 determine the sequence-specificity of DNA binding, whereas the function of ZF1 remains elusive. Three X-ray structures are described here for wild-type -KTS isoform ZF1-4 in complex with its cognate DNA sequence. We observed four unique ZF1 conformations. First, like ZF2-4, ZF1 can be positioned continuously in the DNA major groove forming a 'near-cognate' complex. Second, while ZF2-4 make base-specific interactions with one DNA molecule, ZF1 can interact with a second DNA molecule (or, presumably, two regions of the same DNA molecule). Third, ZF1 can intercalate at the joint of two tail-to-head DNA molecules. If such intercalation occurs on a continuous DNA molecule, it would kink the DNA at the ZF1 binding site. Fourth, two ZF1 units can dimerize. Furthermore, we examined a Denys-Drash syndrome-associated ZF1 mutation (methionine at position 342 is replaced by arginine). This mutation enhances WT1 affinity for a guanine base. X-ray crystallography of the mutant in complex with its preferred sequence revealed the interactions responsible for this affinity change. These results provide insight into the mechanisms of action of WT1, and clarify the fact that ZF1 plays a role in determining sequence specificity of this critical transcription factor.