Summary information and primary citation
- PDB-id
- 6bsg; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- viral protein-DNA-RNA-inhibitor
- Method
- X-ray (2.44 Å)
- Summary
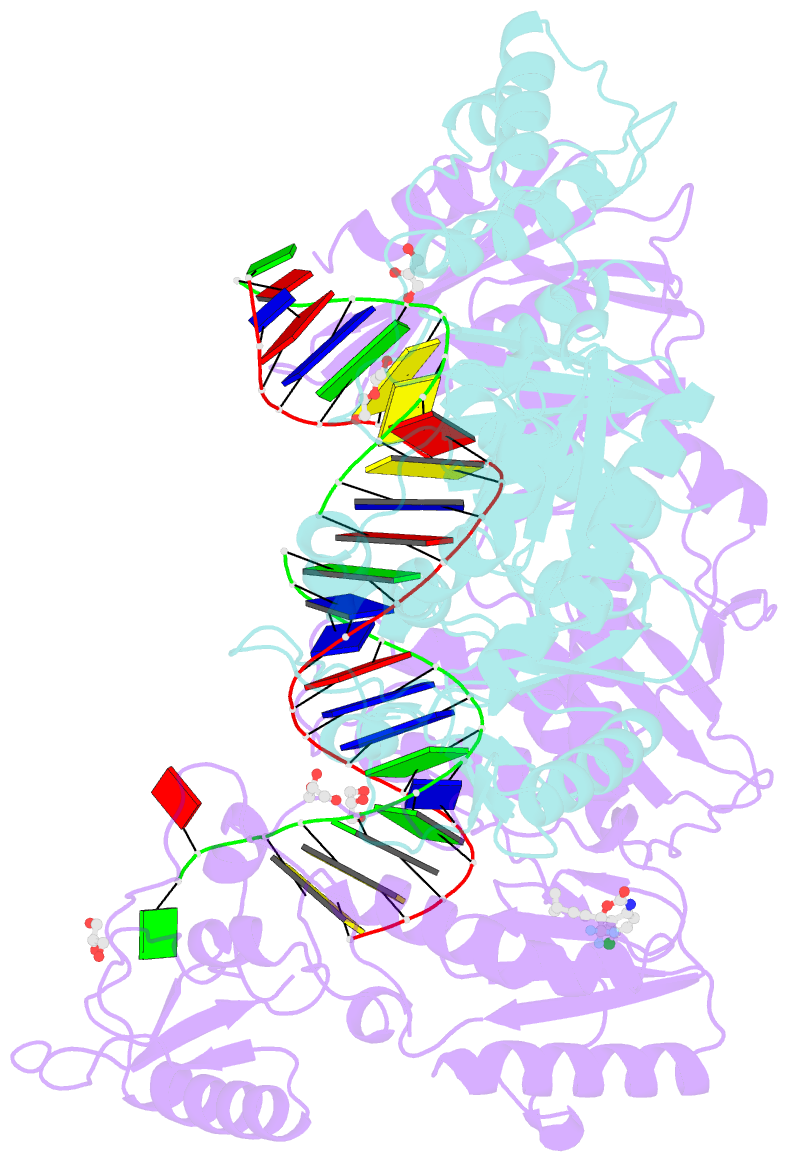
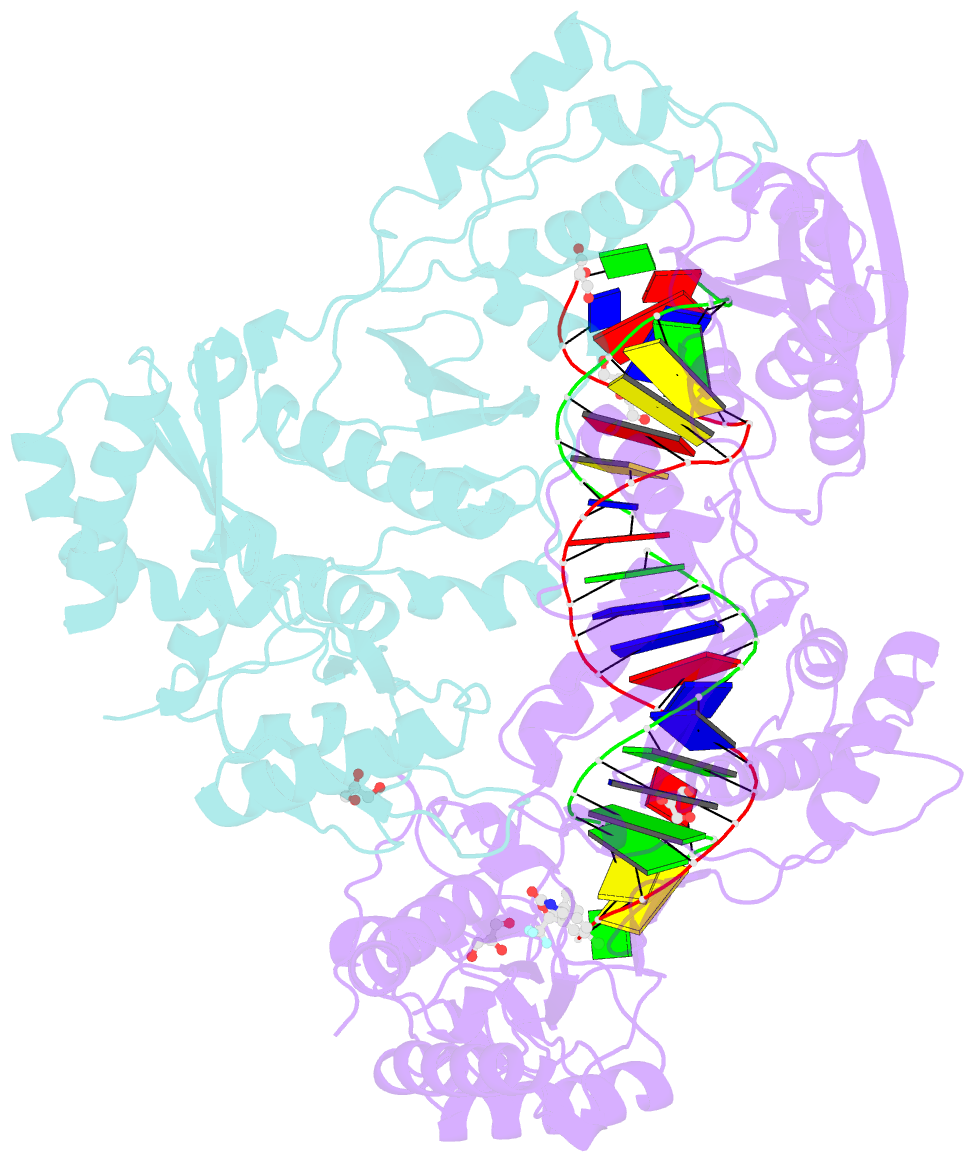
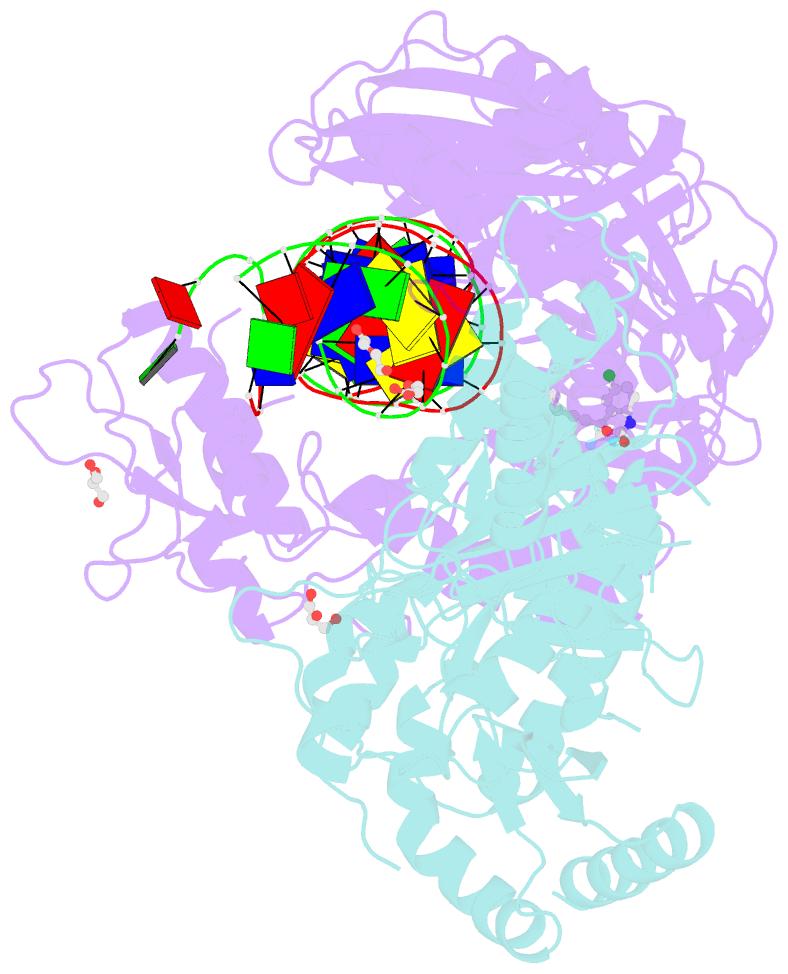
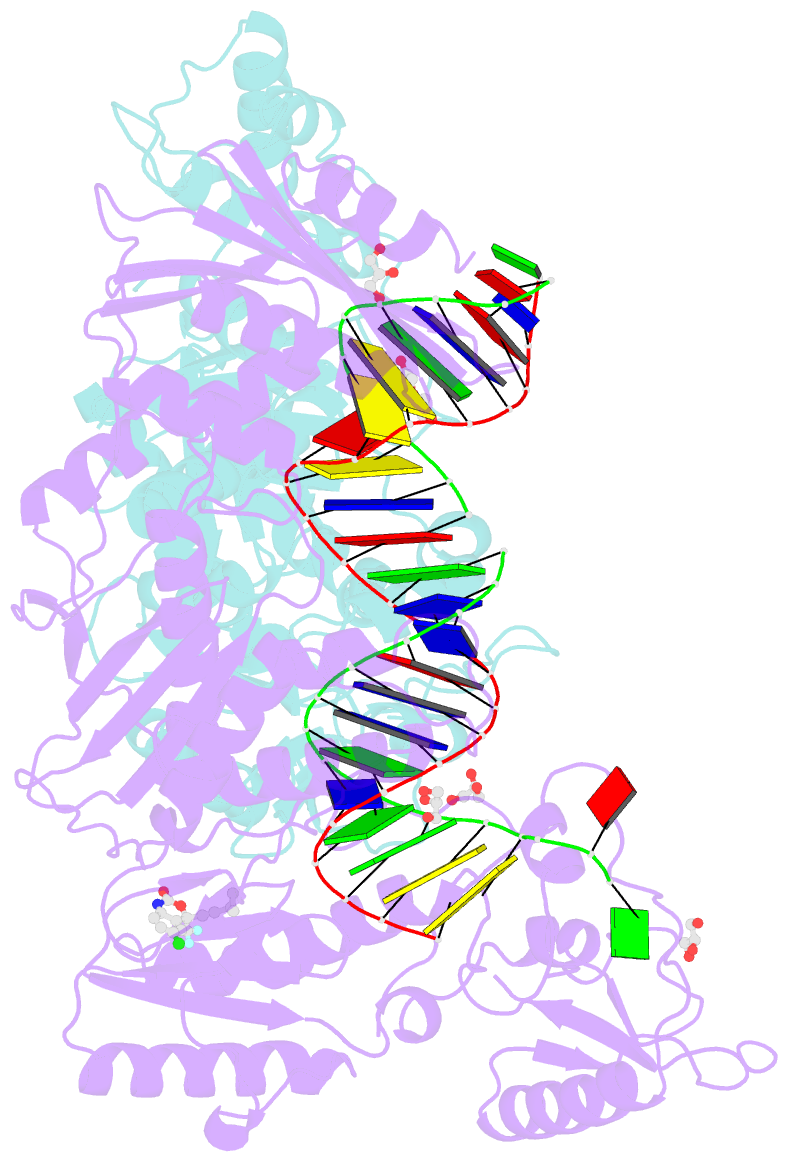
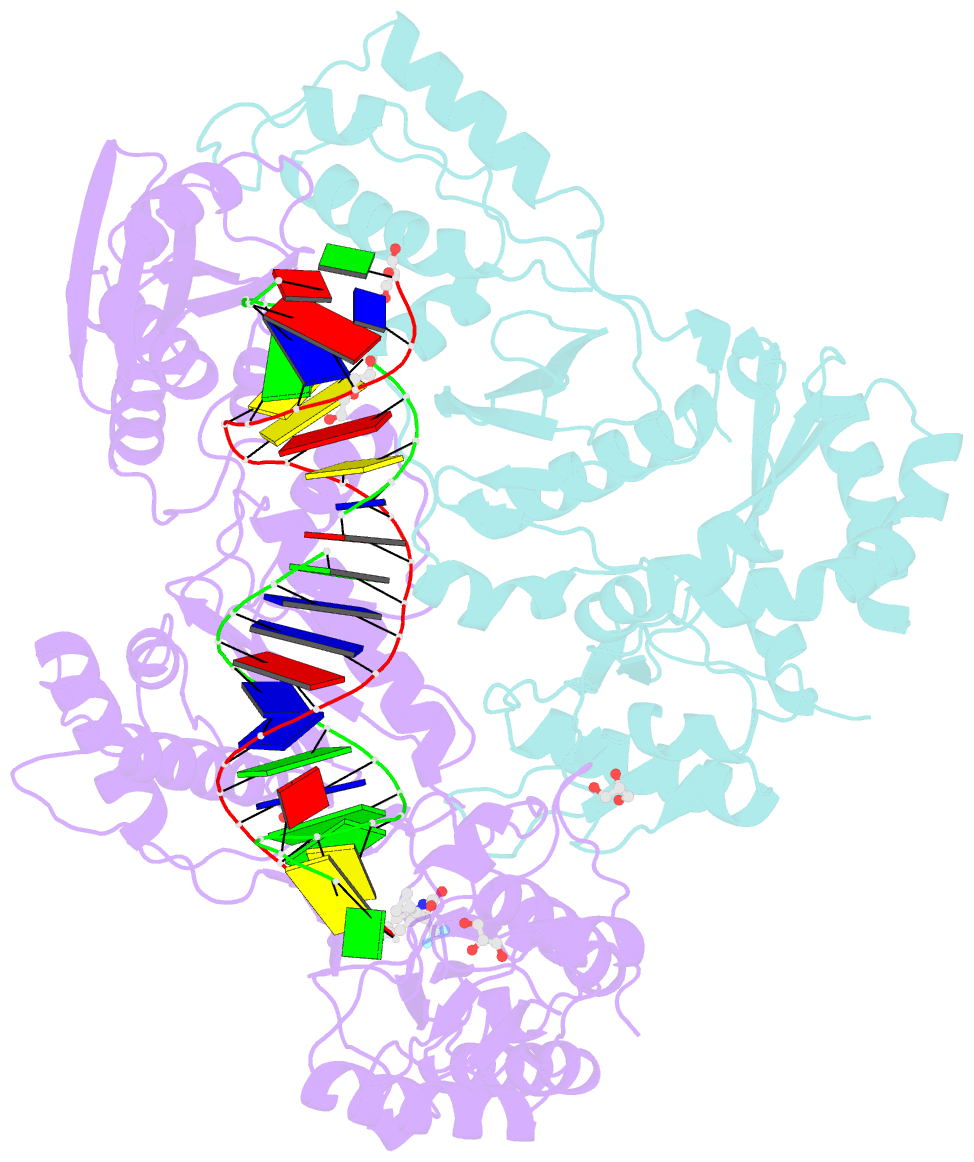
- Structure of hiv-1 rt complexed with RNA-DNA hybrid in an RNA hydrolysis-off mode
- Reference
- Tian L, Kim MS, Li H, Wang J, Yang W (2018): "Structure of HIV-1 reverse transcriptase cleaving RNA in an RNA/DNA hybrid." Proc. Natl. Acad. Sci. U.S.A., 115, 507-512. doi: 10.1073/pnas.1719746115.
- Abstract
- HIV-1 reverse transcriptase (RT) contains both DNA polymerase and RNase H activities to convert the viral genomic RNA to dsDNA in infected host cells. Here we report the 2.65-Å resolution structure of HIV-1 RT engaging in cleaving RNA in an RNA/DNA hybrid. A preferred substrate sequence is absolutely required to enable the RNA/DNA hybrid to adopt the distorted conformation needed to interact properly with the RNase H active site in RT. Substituting two nucleotides 4 bp upstream from the cleavage site results in scissile-phosphate displacement by 4 Å. We also have determined the structure of HIV-1 RT complexed with an RNase H-resistant polypurine tract sequence, which adopts a rigid structure and is accommodated outside of the nuclease active site. Based on this newly gained structural information and a virtual drug screen, we have identified an inhibitor specific for the viral RNase H but not for its cellular homologs.