Summary information and primary citation
- PDB-id
- 6cbd; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein
- Method
- X-ray (2.203 Å)
- Summary
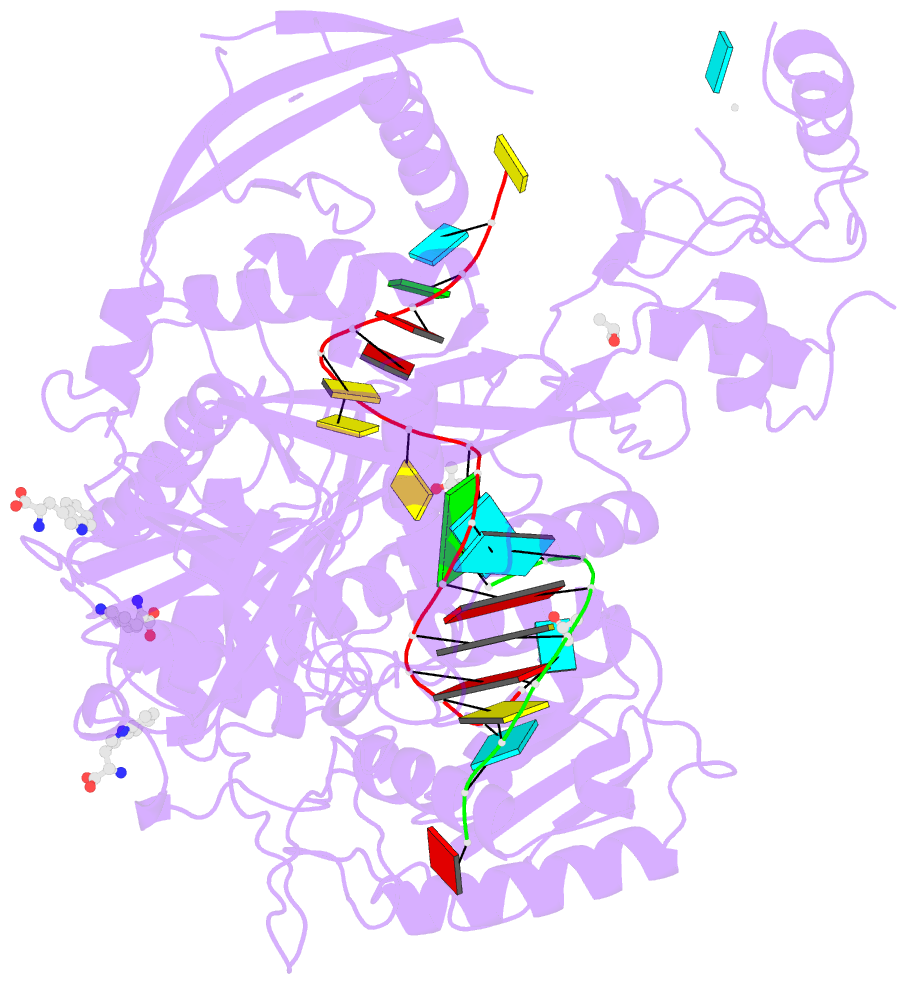
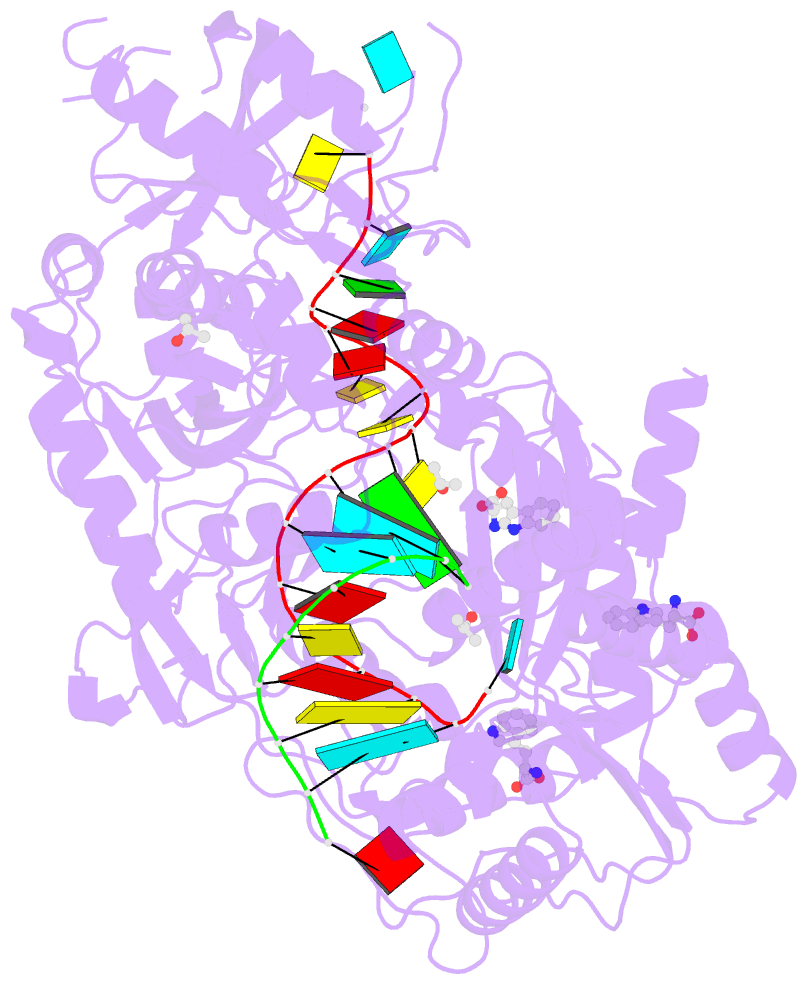
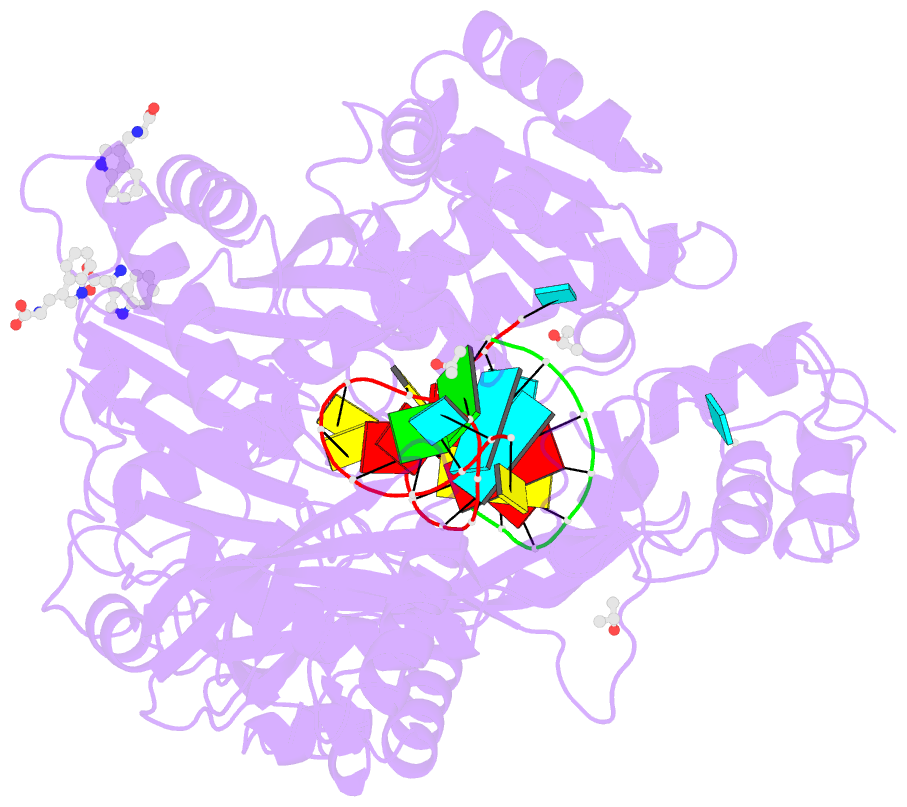
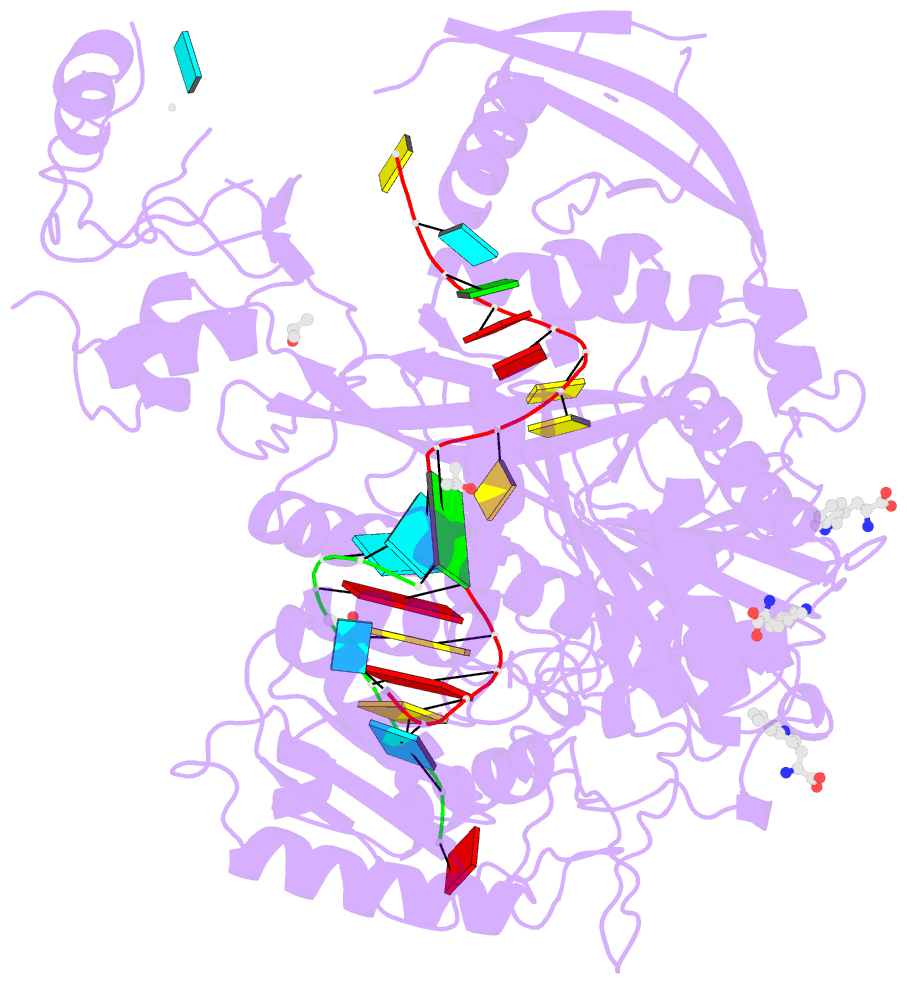
- Crystal structure of human argonaute2 bound to three tryptophans
- Reference
- Sheu-Gruttadauria J, MacRae IJ (2018): "Phase Transitions in the Assembly and Function of Human miRISC." Cell, 173, 946. doi: 10.1016/j.cell.2018.02.051.
- Abstract
- miRISC is a multi-protein assembly that uses microRNAs (miRNAs) to identify mRNAs targeted for repression. Dozens of miRISC-associated proteins have been identified, and interactions between many factors have been examined in detail. However, the physical nature of the complex remains unknown. Here, we show that two core protein components of human miRISC, Argonaute2 (Ago2) and TNRC6B, condense into phase-separated droplets in vitro and in live cells. Phase separation is promoted by multivalent interactions between the glycine/tryptophan (GW)-rich domain of TNRC6B and three evenly spaced tryptophan-binding pockets in the Ago2 PIWI domain. miRISC droplets formed in vitro recruit deadenylation factors and sequester target RNAs from the bulk solution. The condensation of miRISC is accompanied by accelerated deadenylation of target RNAs bound to Ago2. The combined results may explain how miRISC silences mRNAs of varying size and structure and provide experimental evidence that protein-mediated phase separation can facilitate an RNA processing reaction.