Summary information and primary citation
- PDB-id
- 6crm; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (2.19 Å)
- Summary
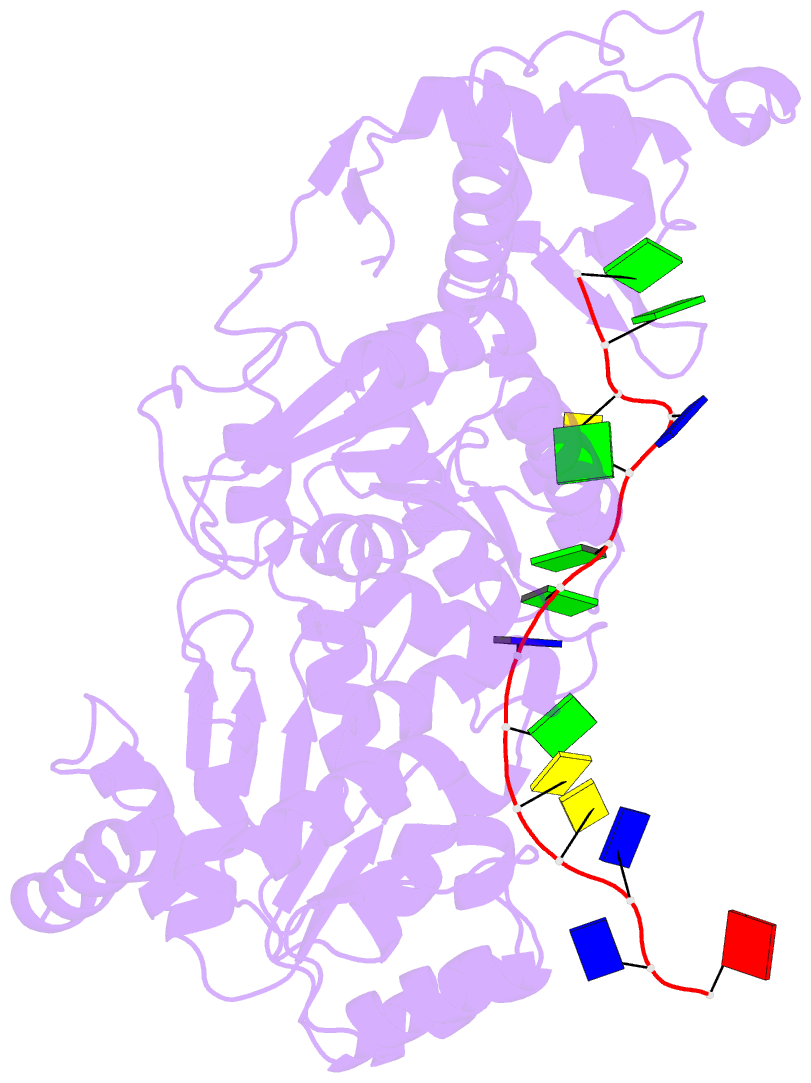
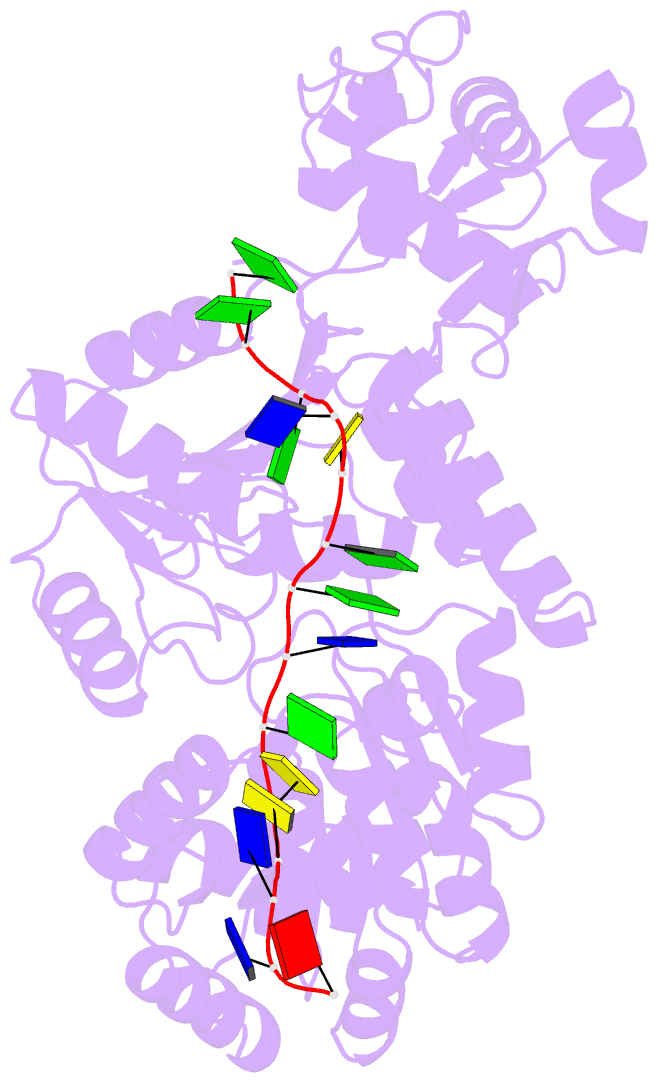
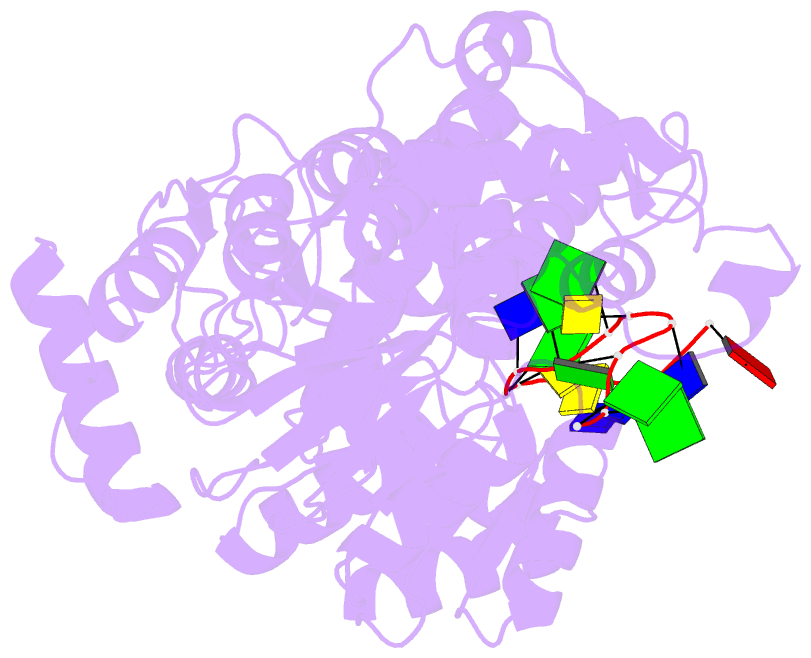
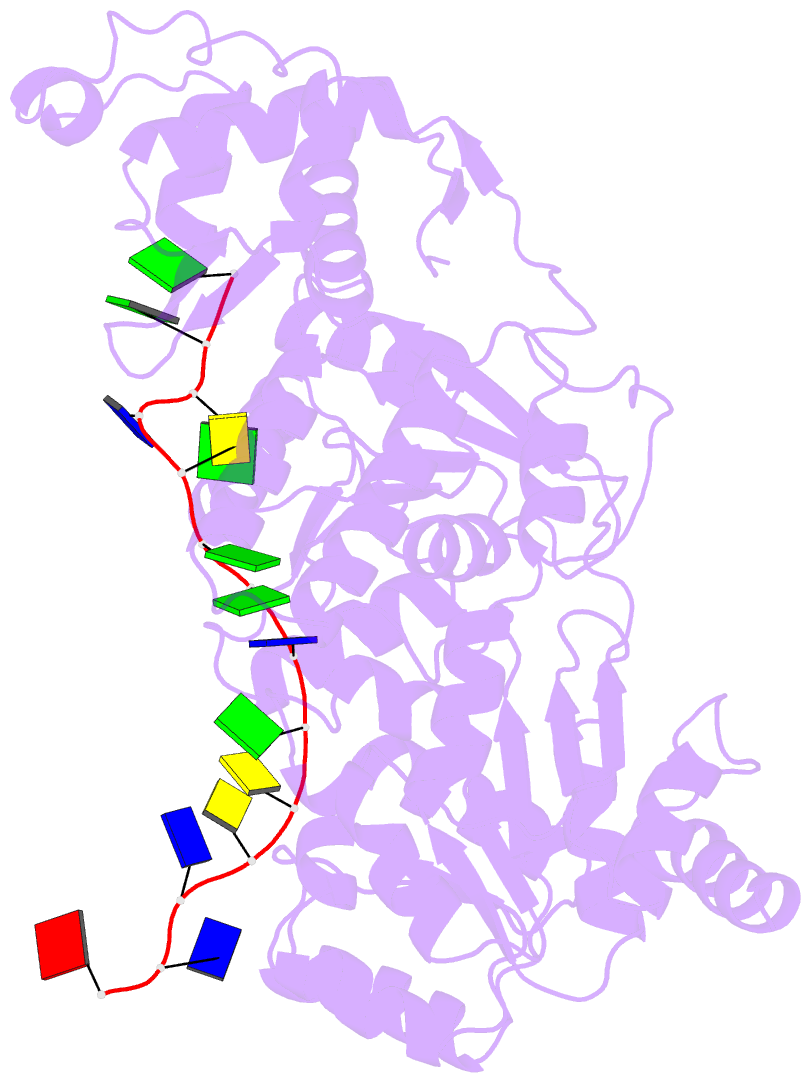
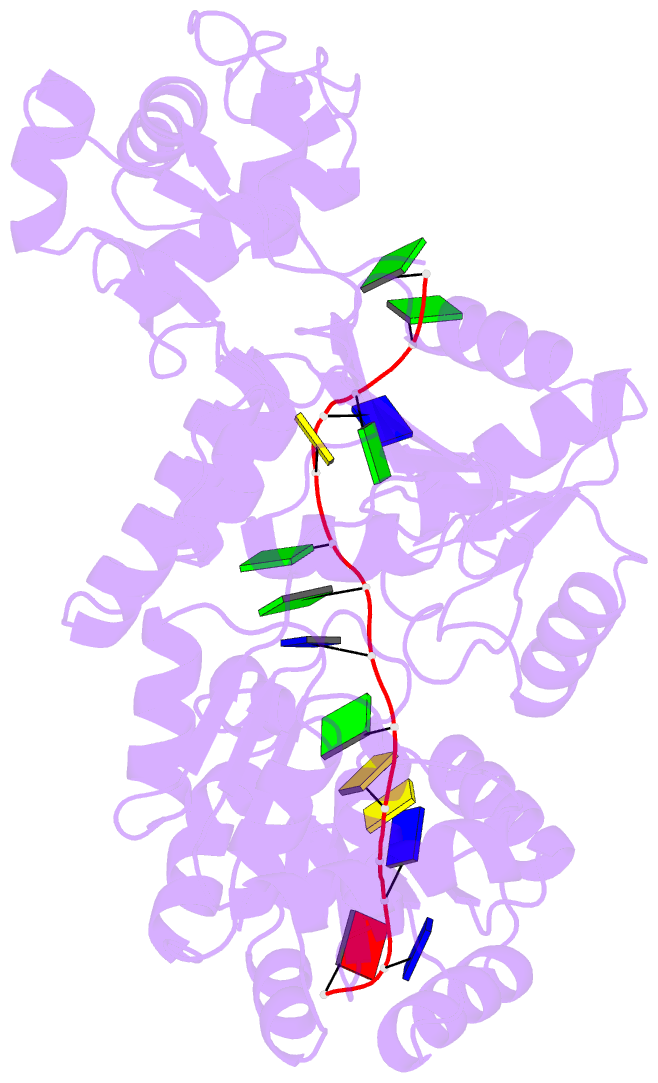
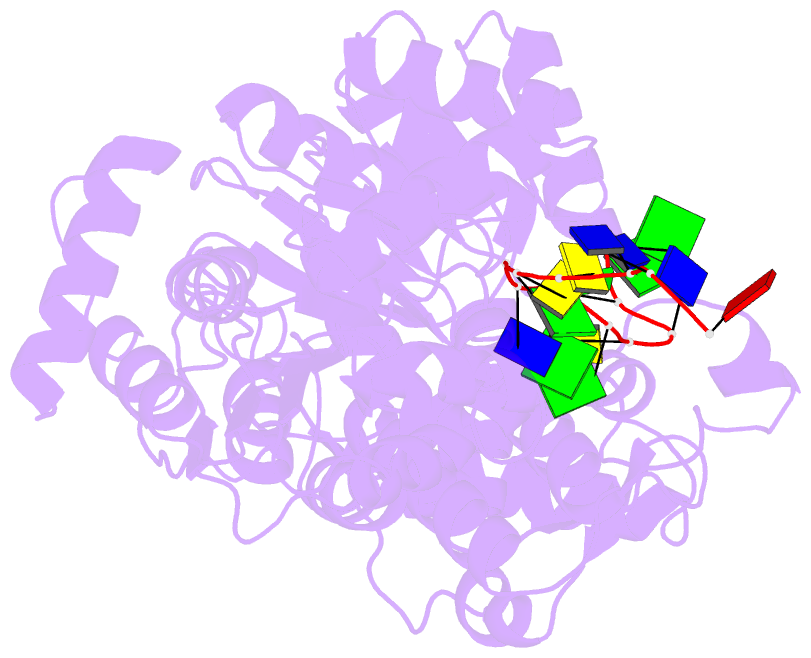
- Crystal structure of recq catalytic core from c. sakazakii bound to an unfolded g-quadruplex
- Reference
- Voter AF, Qiu Y, Tippana R, Myong S, Keck JL (2018): "A guanine-flipping and sequestration mechanism for G-quadruplex unwinding by RecQ helicases." Nat Commun, 9, 4201. doi: 10.1038/s41467-018-06751-8.
- Abstract
- Homeostatic regulation of G-quadruplexes (G4s), four-stranded structures that can form in guanine-rich nucleic acids, requires G4 unwinding helicases. The mechanisms that mediate G4 unwinding remain unknown. We report the structure of a bacterial RecQ DNA helicase bound to resolved G4 DNA. Unexpectedly, a guanine base from the unwound G4 is sequestered within a guanine-specific binding pocket. Disruption of the pocket in RecQ blocks G4 unwinding, but not G4 binding or duplex DNA unwinding, indicating its essential role in structure-specific G4 resolution. A novel guanine-flipping and sequestration model that may be applicable to other G4-resolving helicases emerges from these studies.