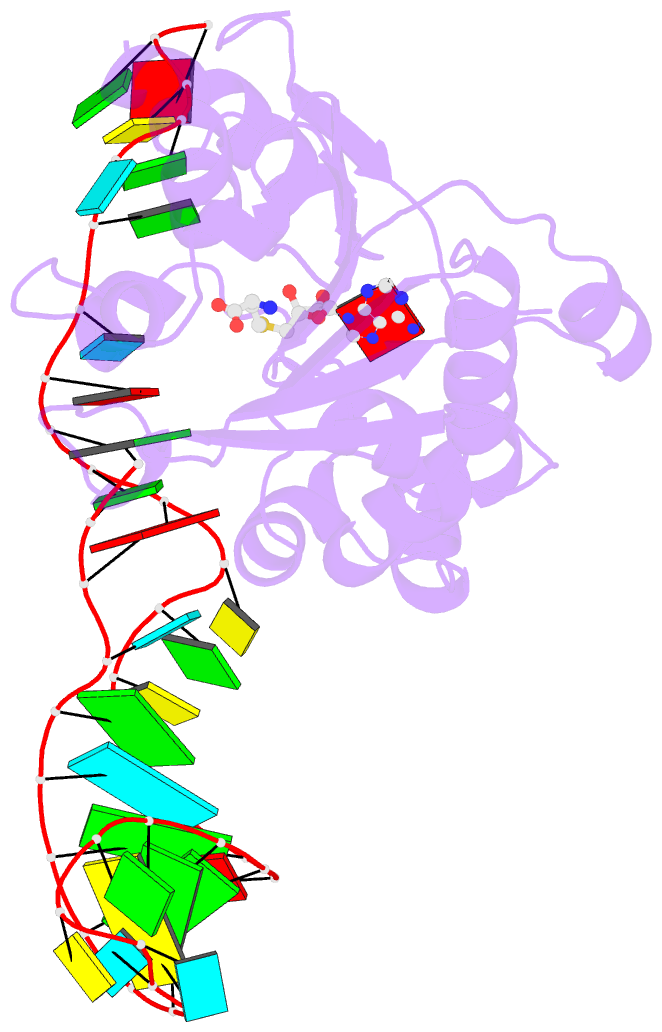
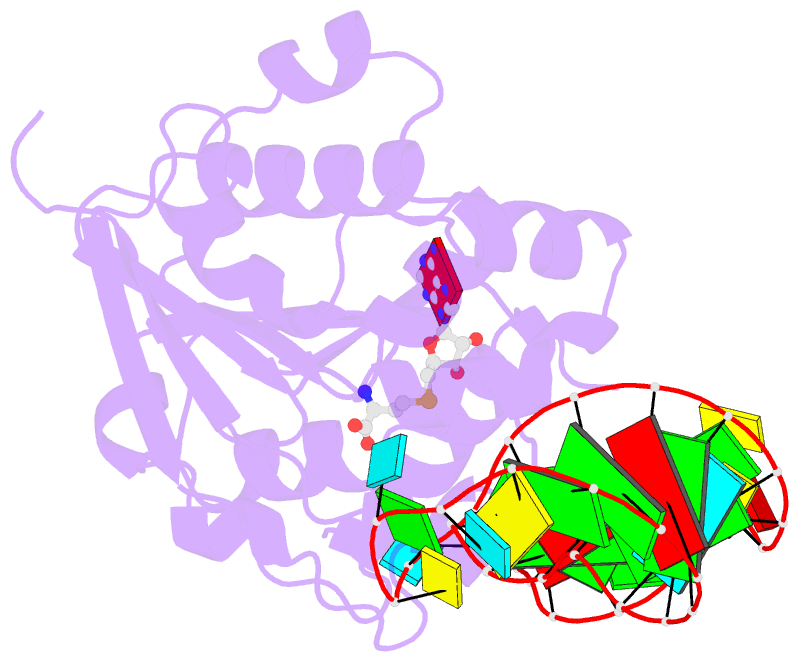
Summary information and primary citation
- PDB-id
- 6dcc; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase-RNA
- Method
- X-ray (2.1 Å)
- Summary
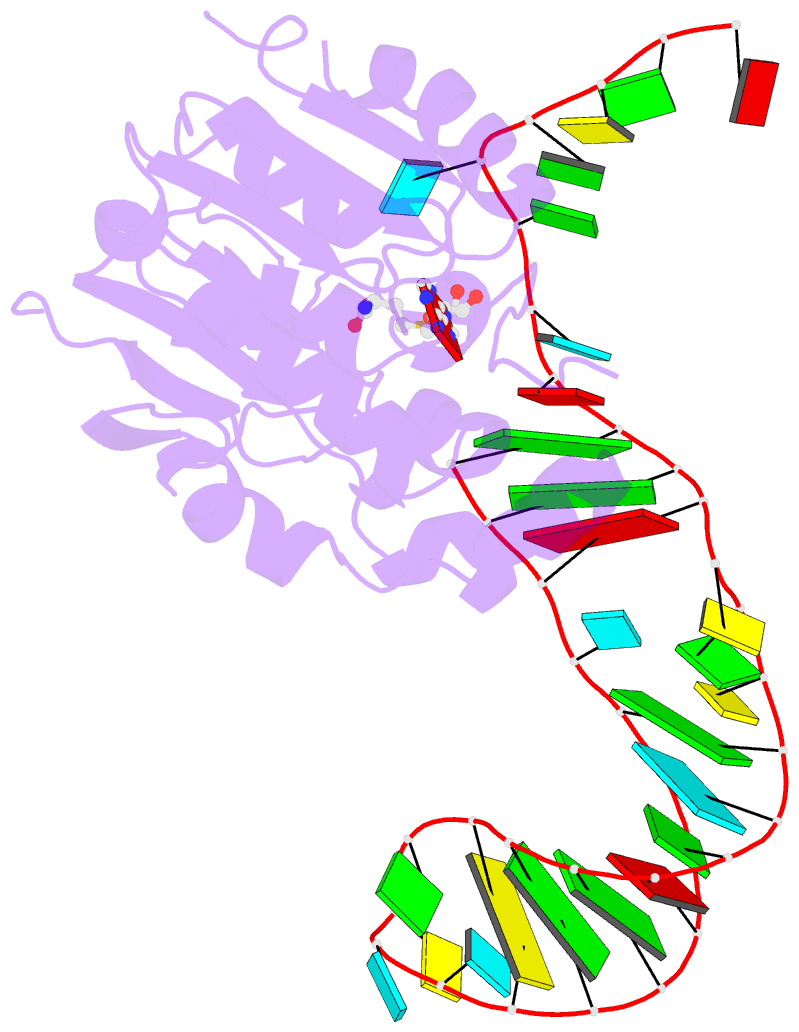
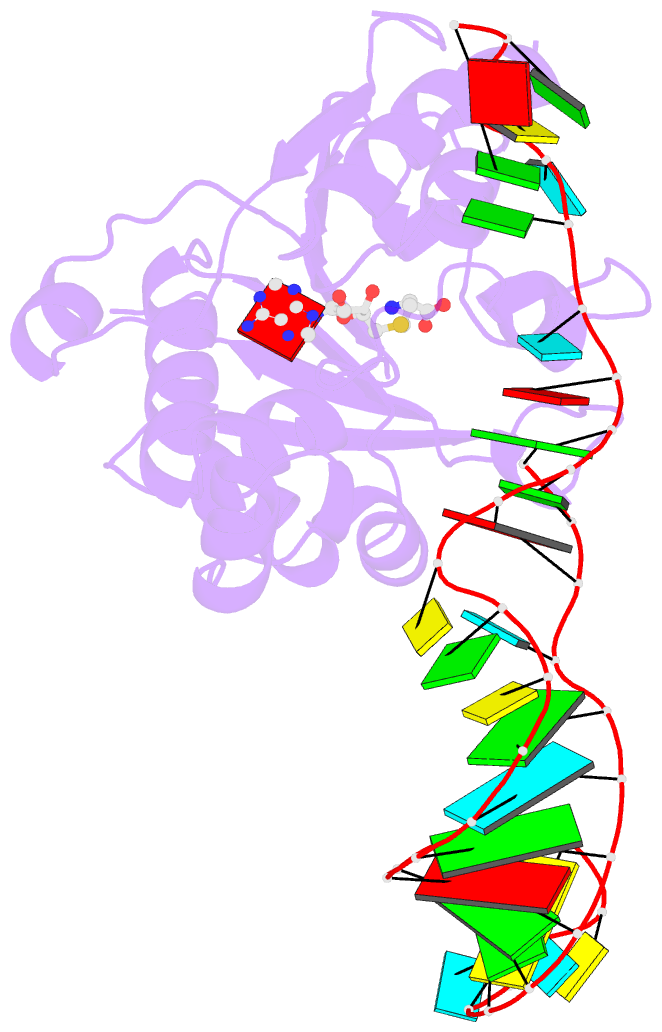
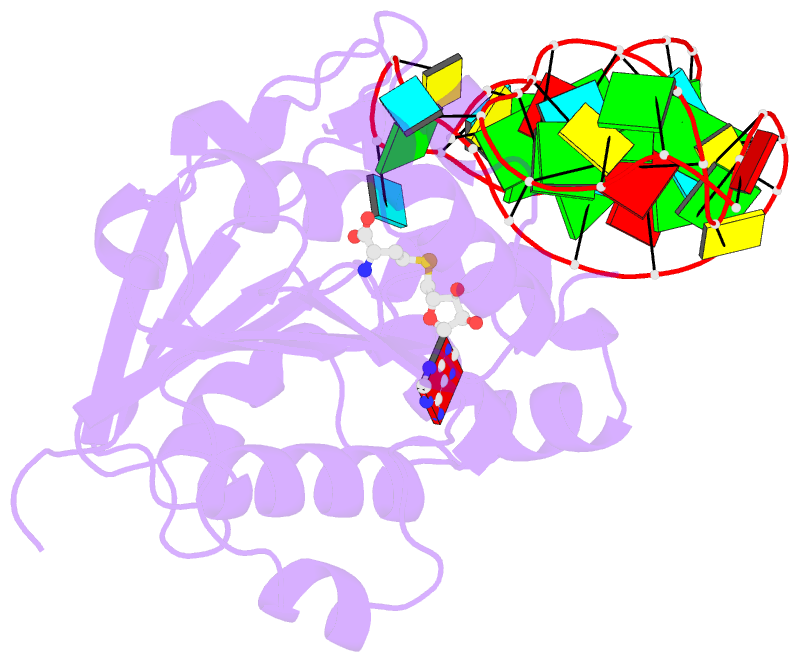
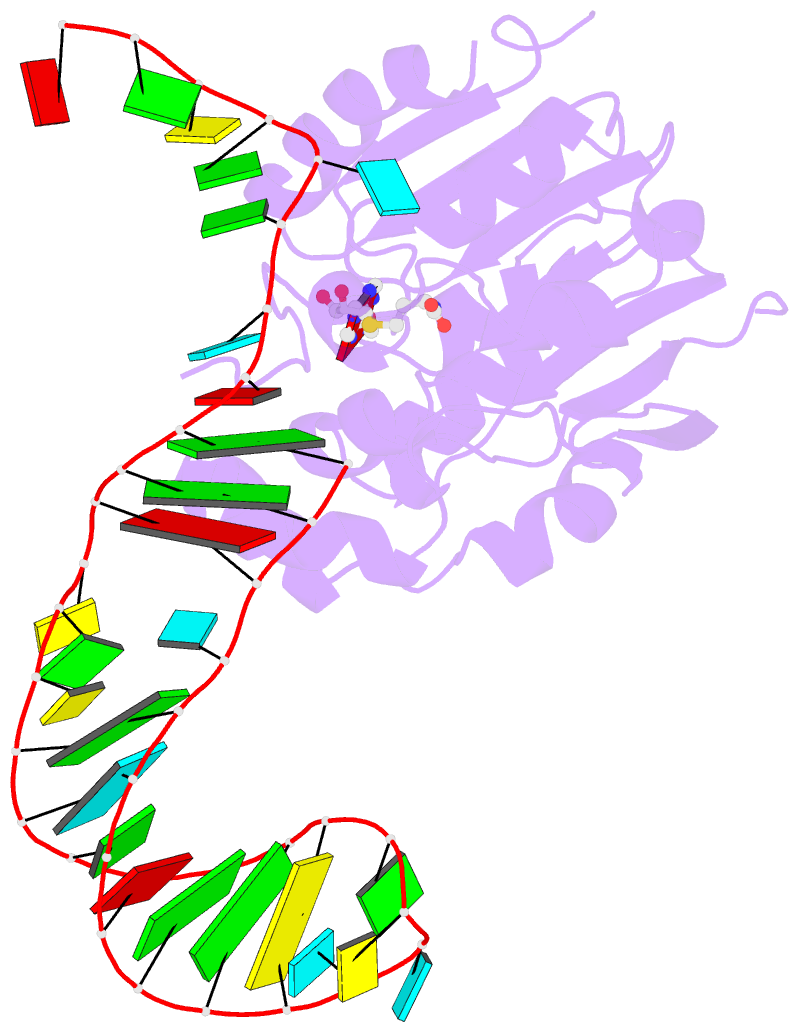
- Structure of methylphosphate capping enzyme methyltransferase domain in complex with 5' end of 7sk RNA
- Reference
- Yang Y, Eichhorn CD, Wang Y, Cascio D, Feigon J (2019): "Structural basis of 7SK RNA 5'-gamma-phosphate methylation and retention by MePCE." Nat. Chem. Biol., 15, 132-140. doi: 10.1038/s41589-018-0188-z.
- Abstract
- Among RNA 5'-cap structures, γ-phosphate monomethylation is unique to a small subset of noncoding RNAs, 7SK and U6 in humans. 7SK is capped by methylphosphate capping enzyme (MePCE), which has a second nonenzymatic role as a core component of the 7SK ribonuclear protein (RNP), an essential regulator of RNA transcription. We report 2.0- and 2.1-Å X-ray crystal structures of the human MePCE methyltransferase domain bound to S-adenosylhomocysteine (SAH) and uncapped or capped 7SK substrates, respectively. 7SK recognition is achieved by protein contacts to a 5'-hairpin-single-stranded RNA region, thus explaining MePCE's specificity for 7SK and U6. The structures reveal SAH and product RNA in a near-transition-state geometry. Unexpectedly, binding experiments showed that MePCE has higher affinity for capped versus uncapped 7SK, and kinetic data support a model of slow product release. This work reveals the molecular mechanism of methyl transfer and 7SK retention by MePCE for subsequent assembly of 7SK RNP.