Summary information and primary citation
- PDB-id
- 6dgd; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-DNA
- Method
- X-ray (2.823 Å)
- Summary
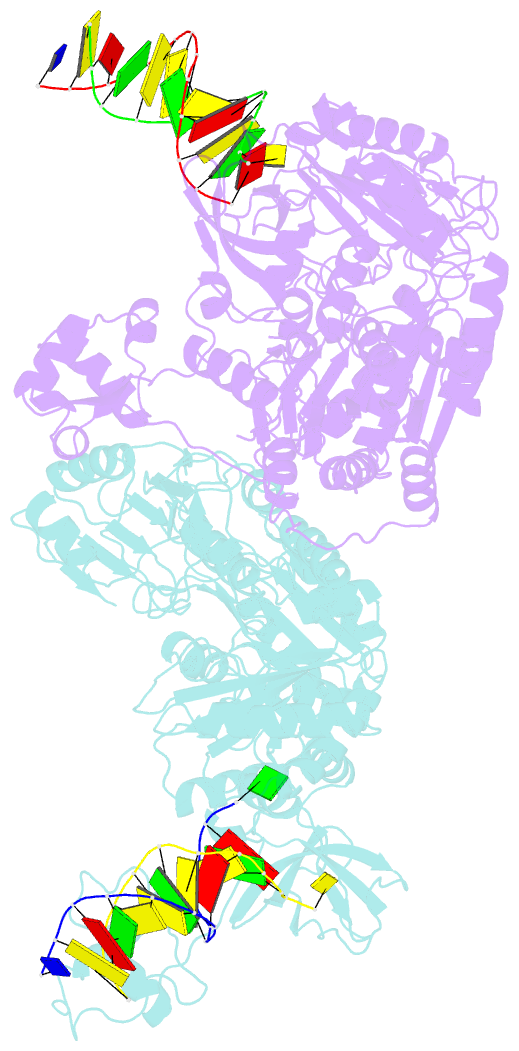
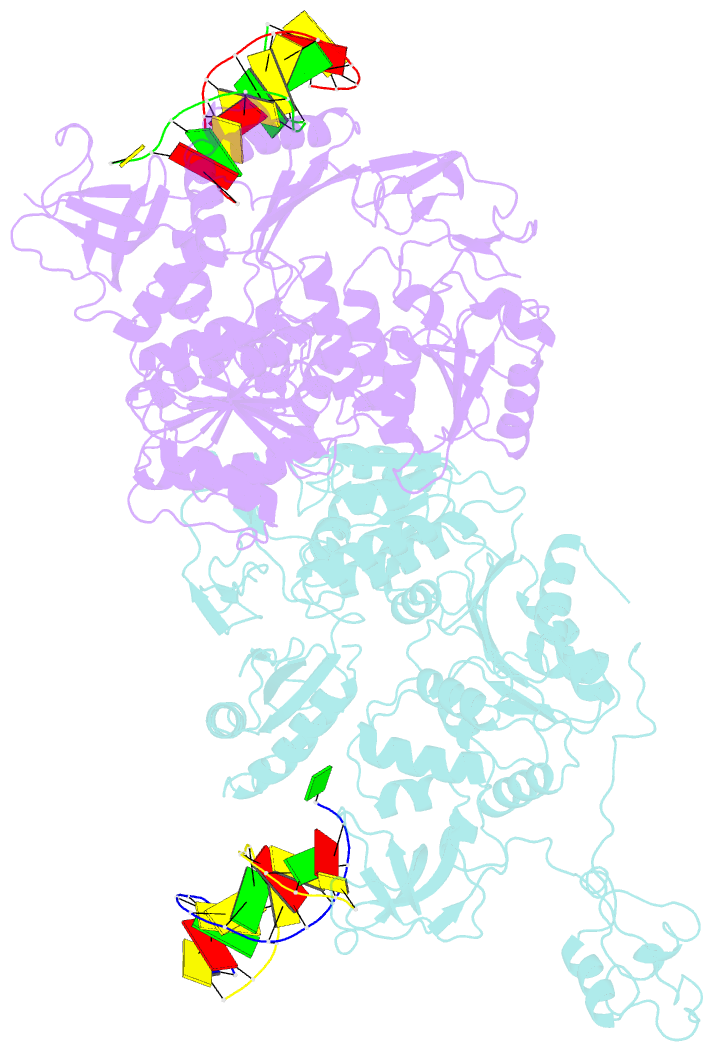
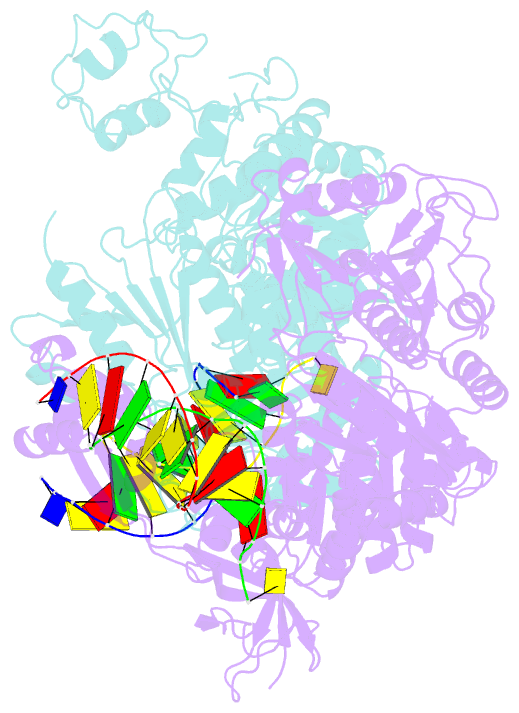
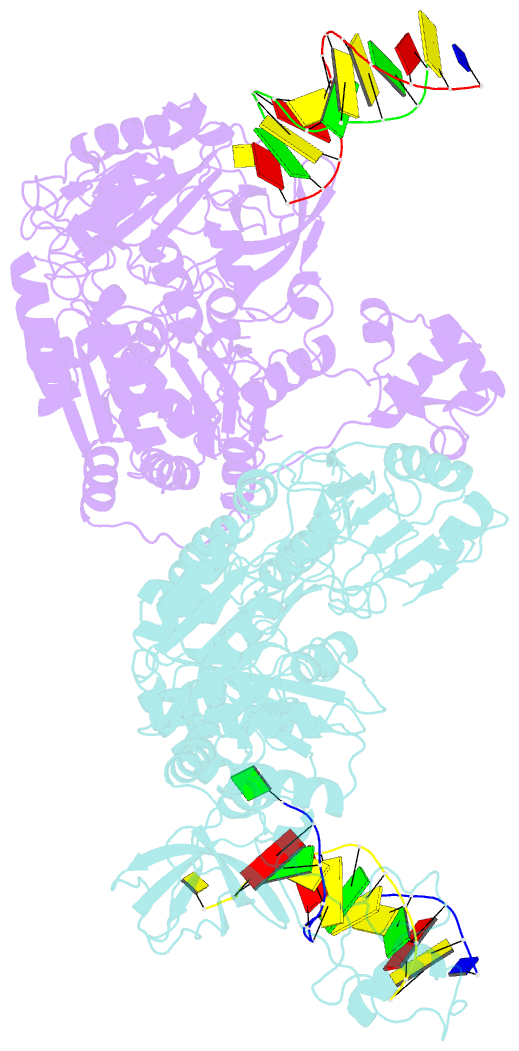
- Pria helicase bound to dsDNA of a DNA replication fork
- Reference
- Windgassen TA, Leroux M, Satyshur KA, Sandler SJ, Keck JL (2018): "Structure-specific DNA replication-fork recognition directs helicase and replication restart activities of the PriA helicase." Proc. Natl. Acad. Sci. U.S.A., 115, E9075-E9084. doi: 10.1073/pnas.1809842115.
- Abstract
- DNA replication restart, the essential process that reinitiates prematurely terminated genome replication reactions, relies on exquisitely specific recognition of abandoned DNA replication-fork structures. The PriA DNA helicase mediates this process in bacteria through mechanisms that remain poorly defined. We report the crystal structure of a PriA/replication-fork complex, which resolves leading-strand duplex DNA bound to the protein. Interaction with PriA unpairs one end of the DNA and sequesters the 3'-most nucleotide from the nascent leading strand into a conserved protein pocket. Cross-linking studies reveal a surface on the winged-helix domain of PriA that binds to parental duplex DNA. Deleting the winged-helix domain alters PriA's structure-specific DNA unwinding properties and impairs its activity in vivo. Our observations lead to a model in which coordinated parental-, leading-, and lagging-strand DNA binding provide PriA with the structural specificity needed to act on abandoned DNA replication forks.