Summary information and primary citation
- PDB-id
- 6dti; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ribosome
- Method
- X-ray (3.54 Å)
- Summary
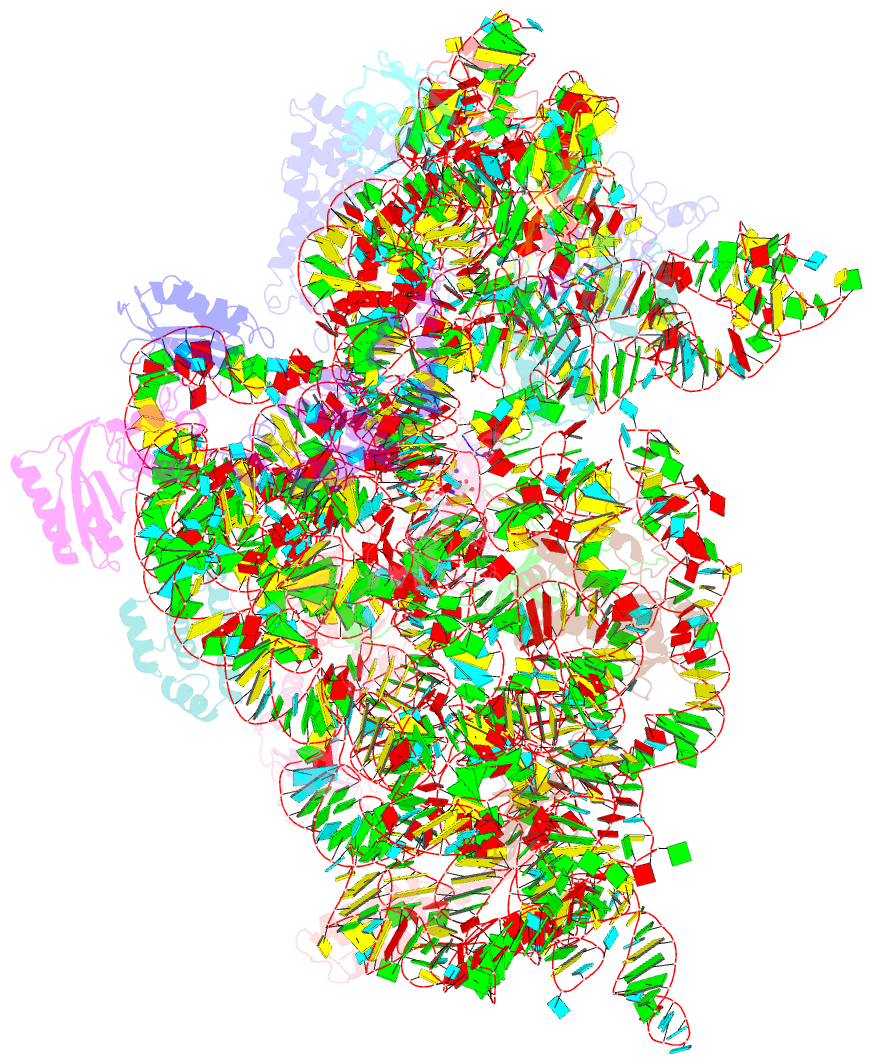
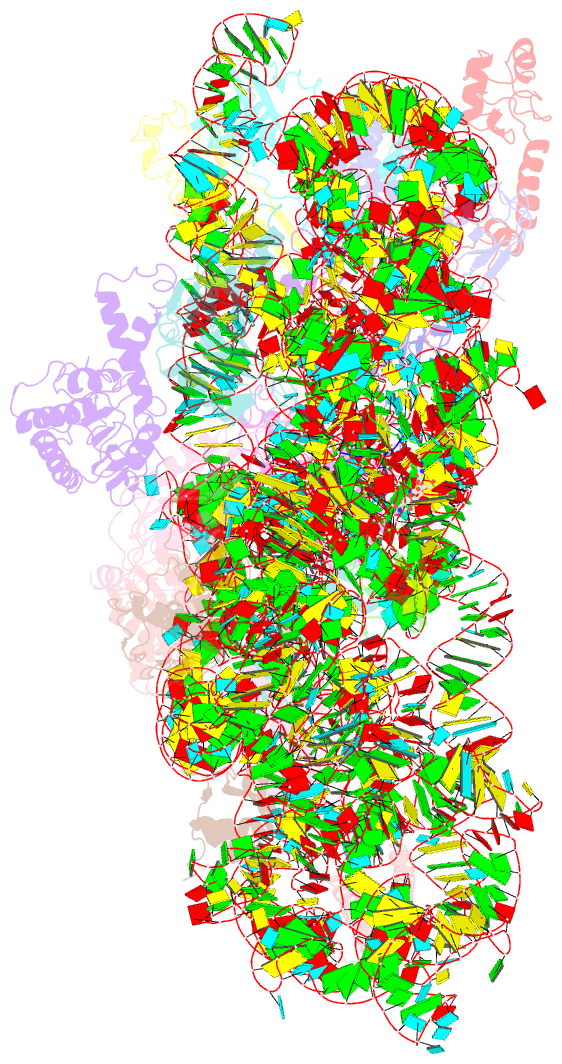
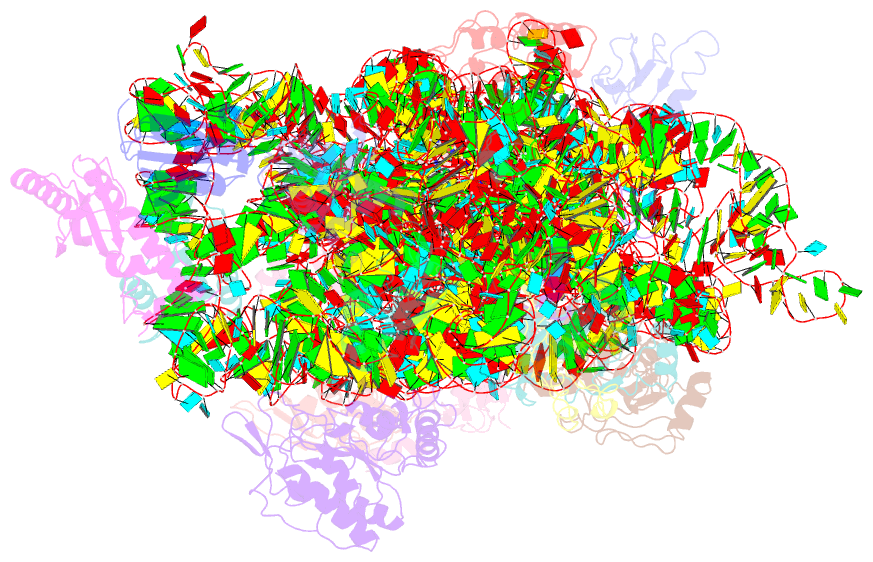
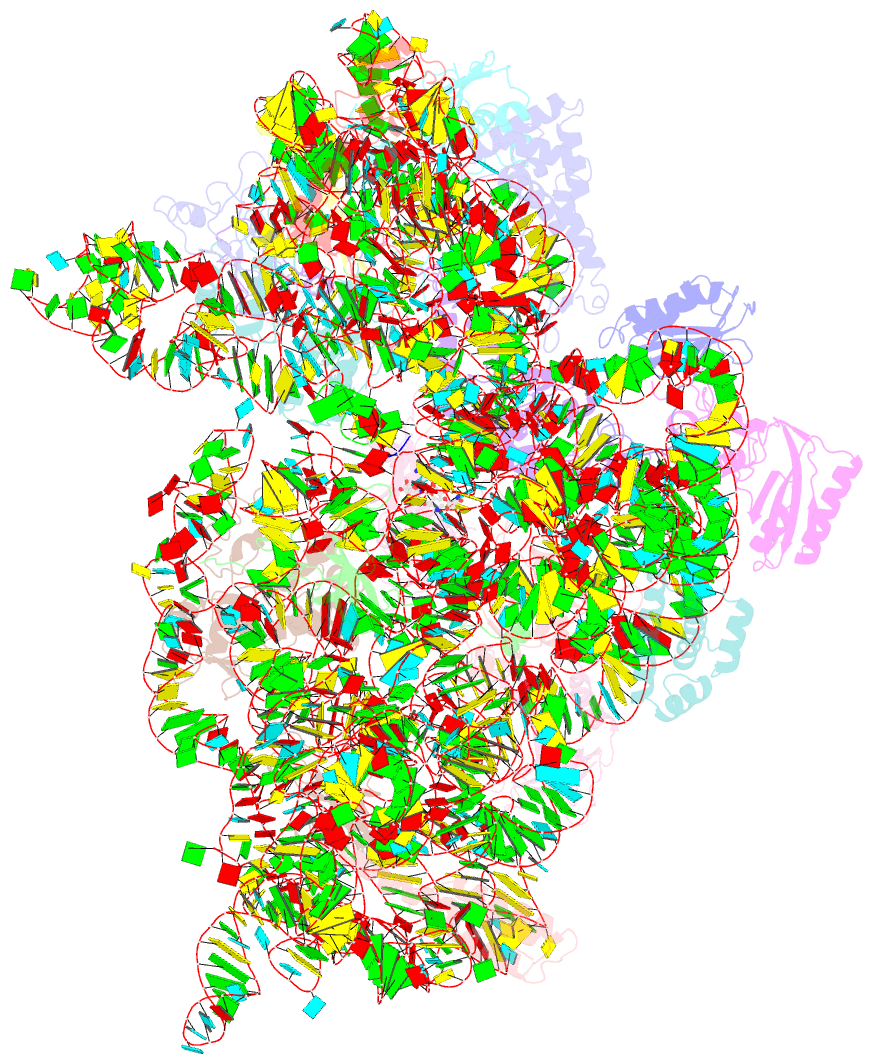
- Structure of the thermus thermophilus 30s ribosomal subunit complexed with an unmodifed anticodon stem loop (asl) of escherichia coli transfer RNA arginine 2 (trnaarg2) bound to an mrna with an cgu-codon in the a-site and paromomycin
- Reference
- Vangaveti S, Cantara WA, Spears JL, DeMirci H, Murphy IV FV, Ranganathan SV, Sarachan KL, Agris PF (2020): "A Structural Basis for Restricted Codon Recognition Mediated by 2-thiocytidine in tRNA Containing a Wobble Position Inosine." J.Mol.Biol., 432, 913-929. doi: 10.1016/j.jmb.2019.12.016.
- Abstract
- Three of six arginine codons (CGU, CGC, and CGA) are decoded by two Escherichia coli tRNAArg isoacceptors. The anticodon stem and loop (ASL) domains of tRNAArg1 and tRNAArg2 both contain inosine and 2-methyladenosine modifications at positions 34 (I34) and 37 (m2A37). tRNAArg1 is also modified from cytidine to 2-thiocytidine at position 32 (s2C32). The s2C32 modification is known to negate wobble codon recognition of the rare CGA codon by an unknown mechanism, while still allowing decoding of CGU and CGC. Substitution of s2C32 for C32 in the Saccharomyces cerevisiae tRNAIleIAU anticodon stem and loop domain (ASL) negates wobble decoding of its synonymous A-ending codon, suggesting that this function of s2C at position 32 is a generalizable property. X-ray crystal structures of variously modified ASLArg1ICG and ASLArg2ICG constructs bound to cognate and wobble codons on the ribosome revealed the disruption of a C32-A38 cross-loop interaction but failed to fully explain the means by which s2C32 restricts I34 wobbling. Computational studies revealed that the adoption of a spatially broad inosine-adenosine base pair at the wobble position of the codon cannot be maintained simultaneously with the canonical ASL U-turn motif. C32-A38 cross-loop interactions are required for stability of the anticodon/codon interaction in the ribosomal A-site.