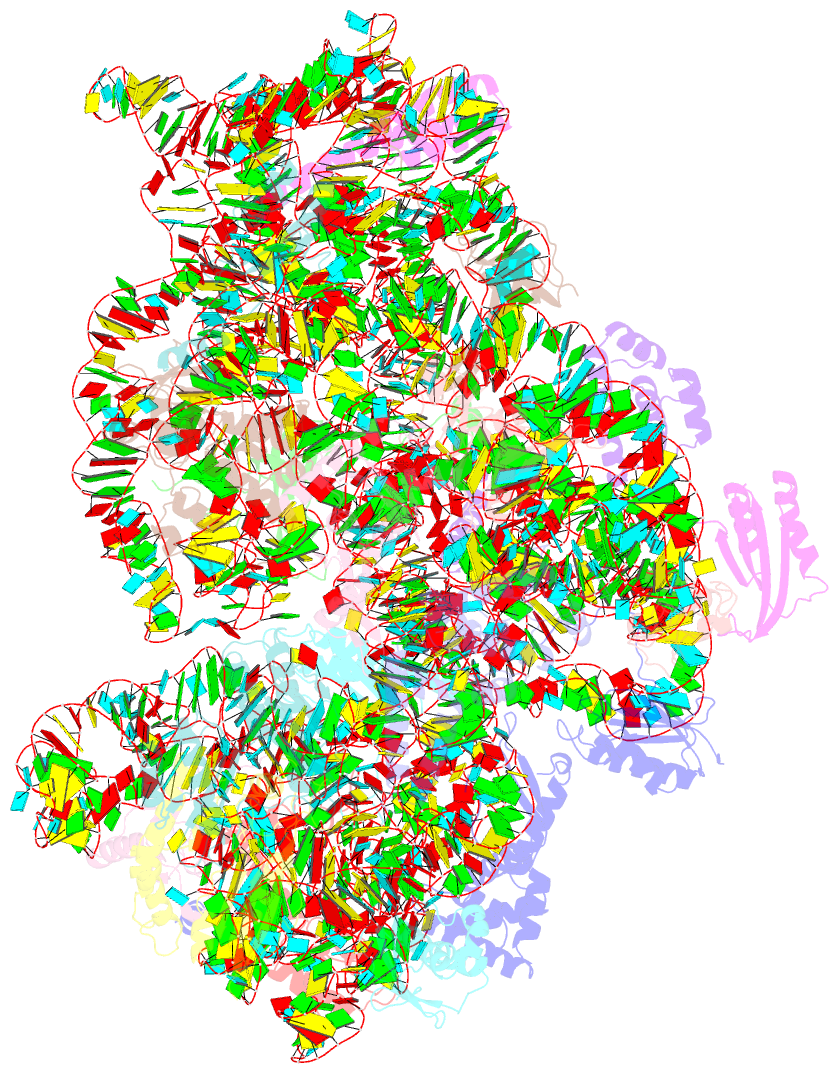
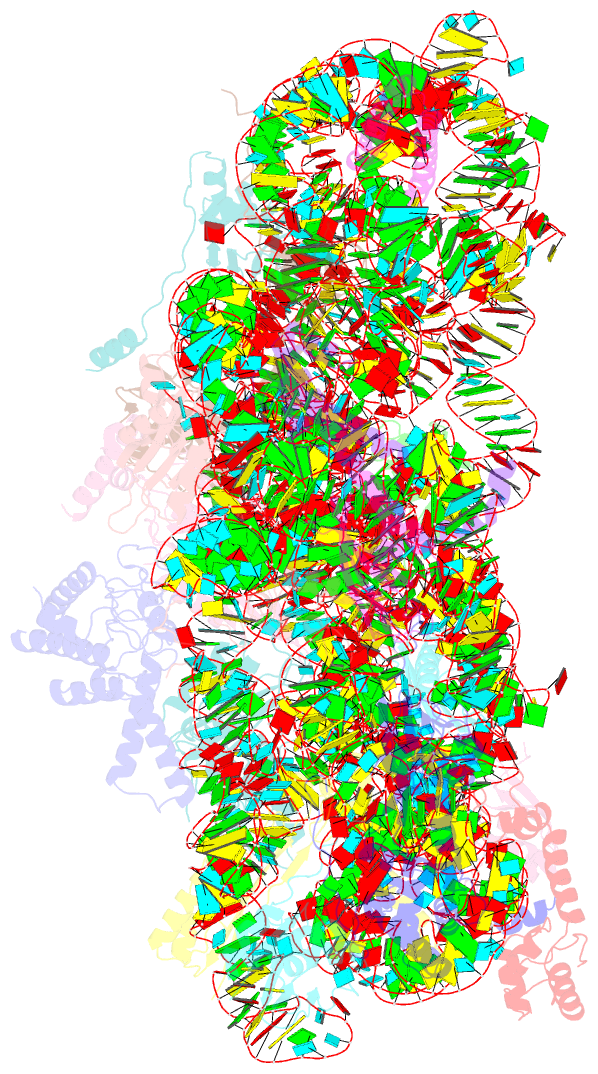
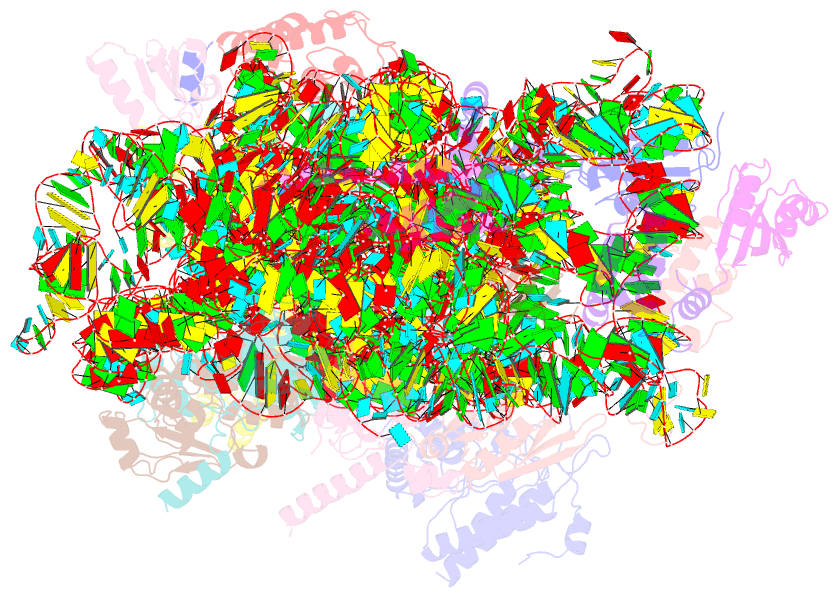
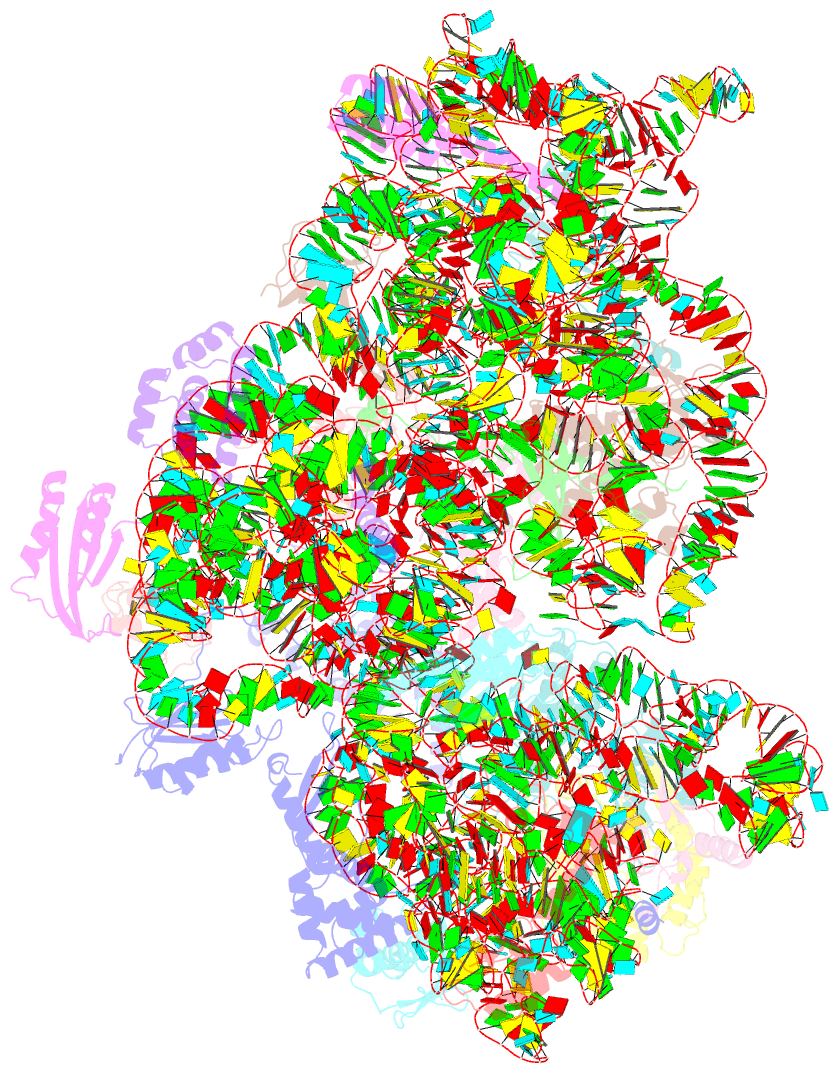
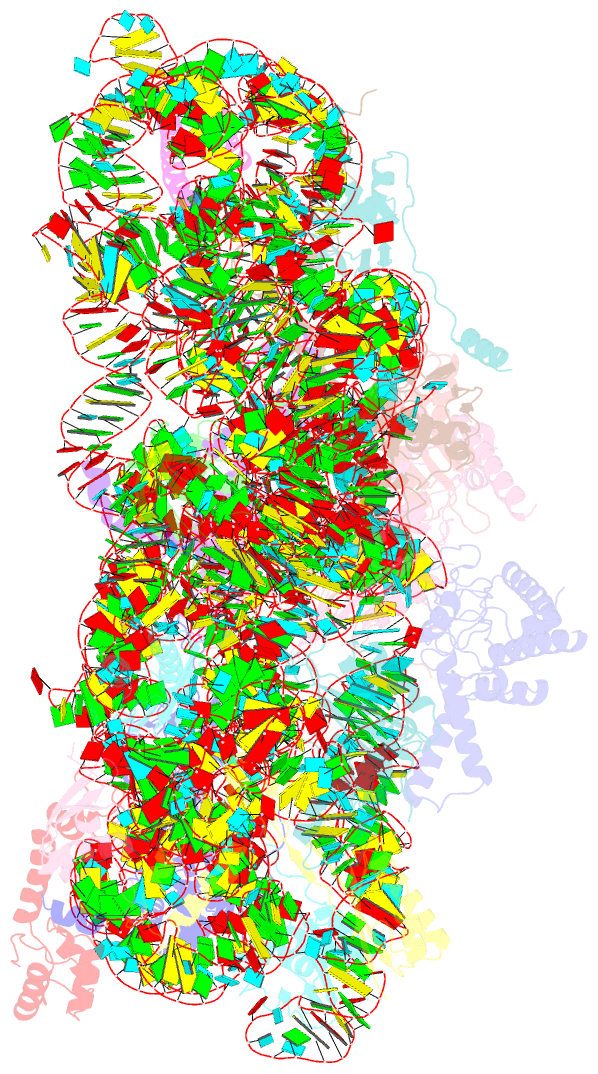
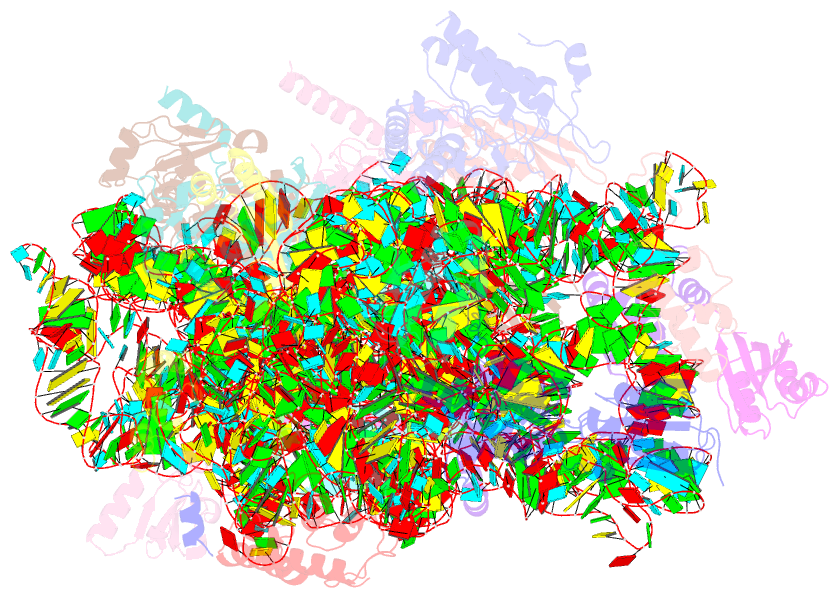
Summary information and primary citation
- PDB-id
- 6dzk; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ribosome
- Method
- cryo-EM (3.6 Å)
- Summary
- cryo-EM structure of mycobacterium smegmatis c(minus) 30s ribosomal subunit with mpy
- Reference
- Li Y, Sharma MR, Koripella RK, Yang Y, Kaushal PS, Lin Q, Wade JT, Gray TA, Derbyshire KM, Agrawal RK, Ojha AK (2018): "Zinc depletion induces ribosome hibernation in mycobacteria." Proc. Natl. Acad. Sci. U.S.A., 115, 8191-8196. doi: 10.1073/pnas.1804555115.
- Abstract
- Bacteria respond to zinc starvation by replacing ribosomal proteins that have the zinc-binding CXXC motif (C+) with their zinc-free (C-) paralogues. Consequences of this process beyond zinc homeostasis are unknown. Here, we show that the C- ribosome in Mycobacterium smegmatis is the exclusive target of a bacterial protein Y homolog, referred to as mycobacterial-specific protein Y (MPY), which binds to the decoding region of the 30S subunit, thereby inactivating the ribosome. MPY binding is dependent on another mycobacterial protein, MPY recruitment factor (MRF), which is induced on zinc depletion, and interacts with C- ribosomes. MPY binding confers structural stability to C- ribosomes, promoting survival of growth-arrested cells under zinc-limiting conditions. Binding of MPY also has direct influence on the dynamics of aminoglycoside-binding pockets of the C- ribosome to inhibit binding of these antibiotics. Together, our data suggest that zinc limitation leads to ribosome hibernation and aminoglycoside resistance in mycobacteria. Furthermore, our observation of the expression of the proteins of C- ribosomes in Mycobacterium tuberculosis in a mouse model of infection suggests that ribosome hibernation could be relevant in our understanding of persistence and drug tolerance of the pathogen encountered during chemotherapy of TB.