Summary information and primary citation
- PDB-id
- 6e4p; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein-RNA
- Method
- X-ray (1.949 Å)
- Summary
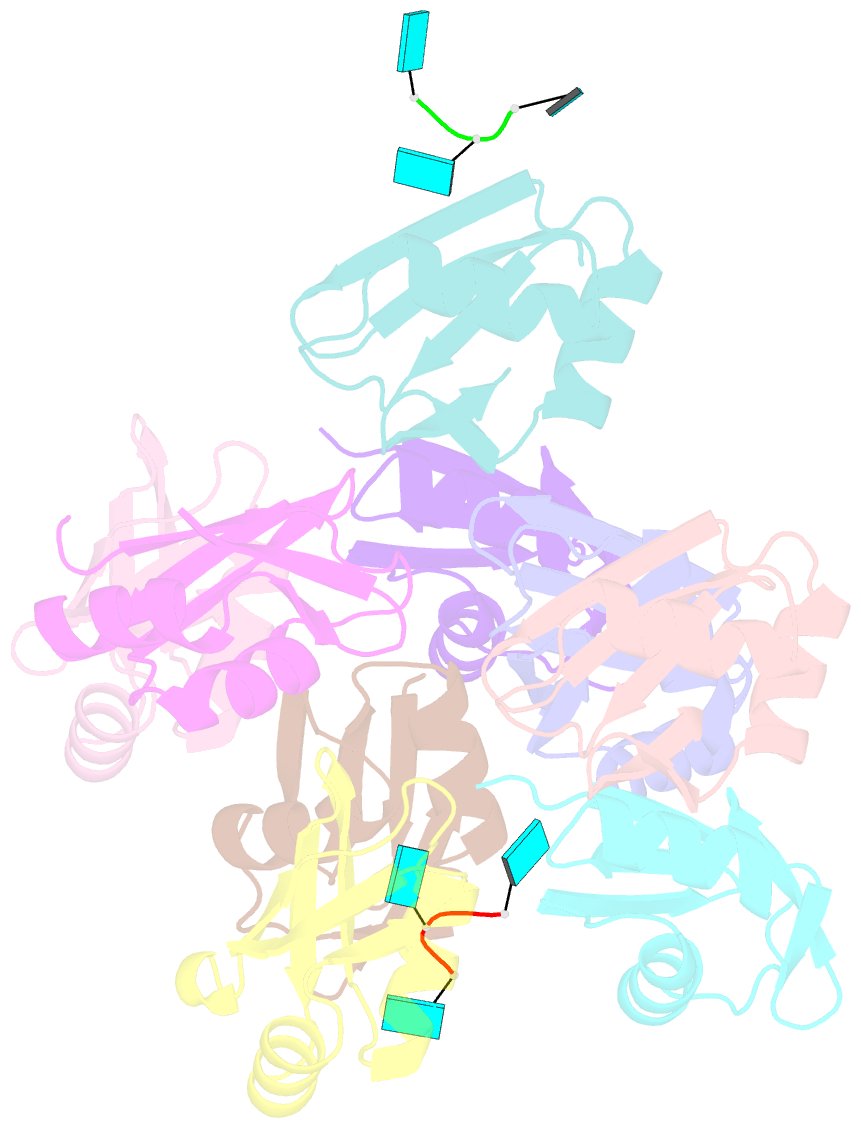
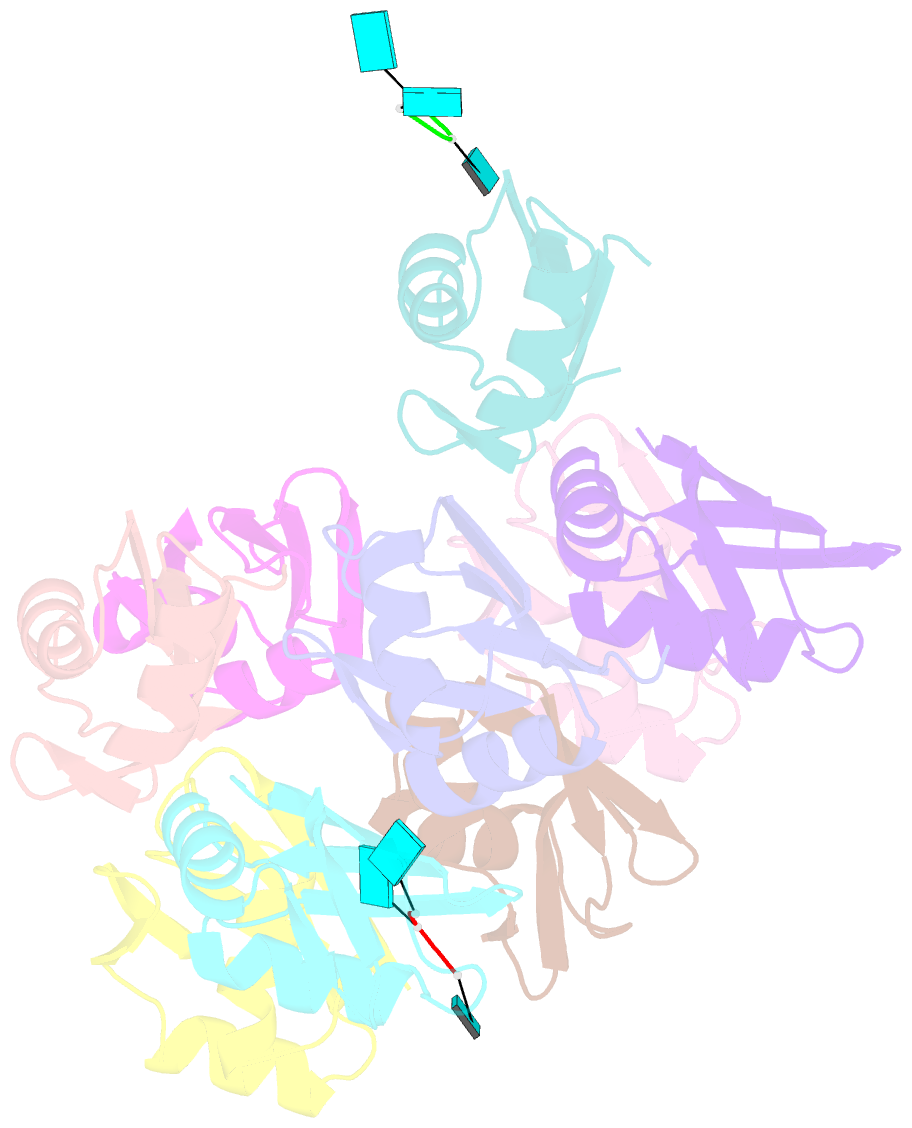
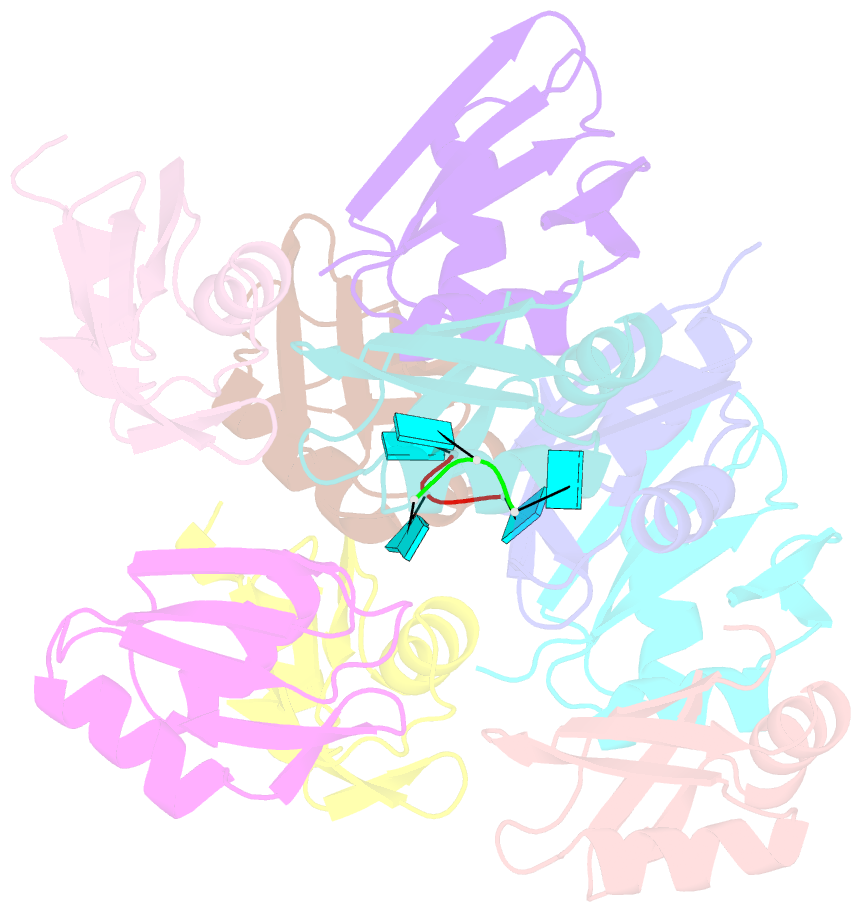
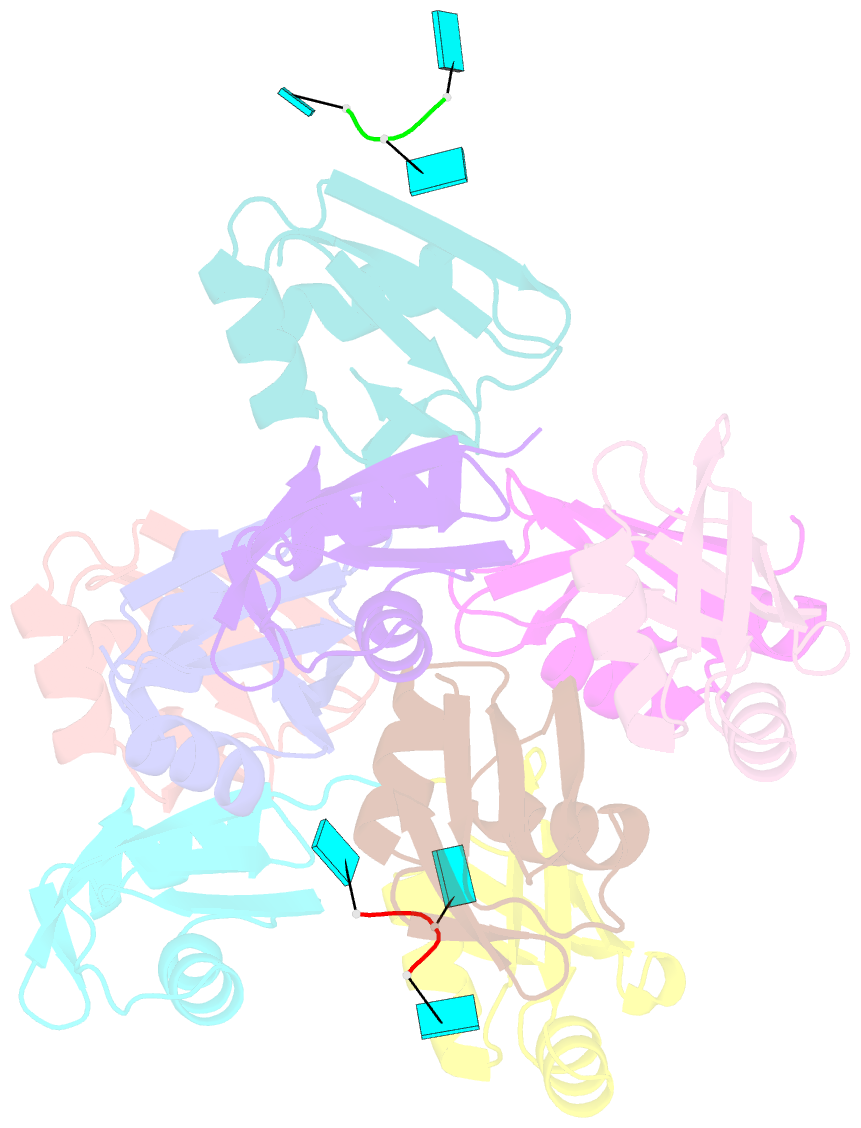
- Structure of the t. brucei rrm domain in complex with RNA
- Reference
- Travis B, Shaw PLR, Liu B, Ravindra K, Iliff H, Al-Hashimi HM, Schumacher MA (2019): "The RRM of the kRNA-editing protein TbRGG2 uses multiple surfaces to bind and remodel RNA." Nucleic Acids Res., 47, 2130-2142. doi: 10.1093/nar/gky1259.
- Abstract
- Kinetoplastid RNA (kRNA) editing takes place in the mitochondria of kinetoplastid protists and creates translatable mRNAs by uridine insertion/deletion. Extensively edited (pan-edited) transcripts contain quadruplex forming guanine stretches, which must be remodeled to promote uridine insertion/deletion. Here we show that the RRM domain of the essential kRNA-editing factor TbRGG2 binds poly(G) and poly(U) RNA and can unfold both. A region C-terminal to the RRM mediates TbRGG2 dimerization, enhancing RNA binding. A RRM-U4 RNA structure reveals a unique RNA-binding mechanism in which the two RRMs of the dimer employ aromatic residues outside the canonical RRM RNA-binding motifs to encase and wrench open the RNA, while backbone atoms specify the uridine bases. Notably, poly(G) RNA is bound via a different binding surface. Thus, these data indicate that TbRGG2 RRM can bind and remodel several RNA substrates suggesting how it might play multiple roles in the kRNA editing process.