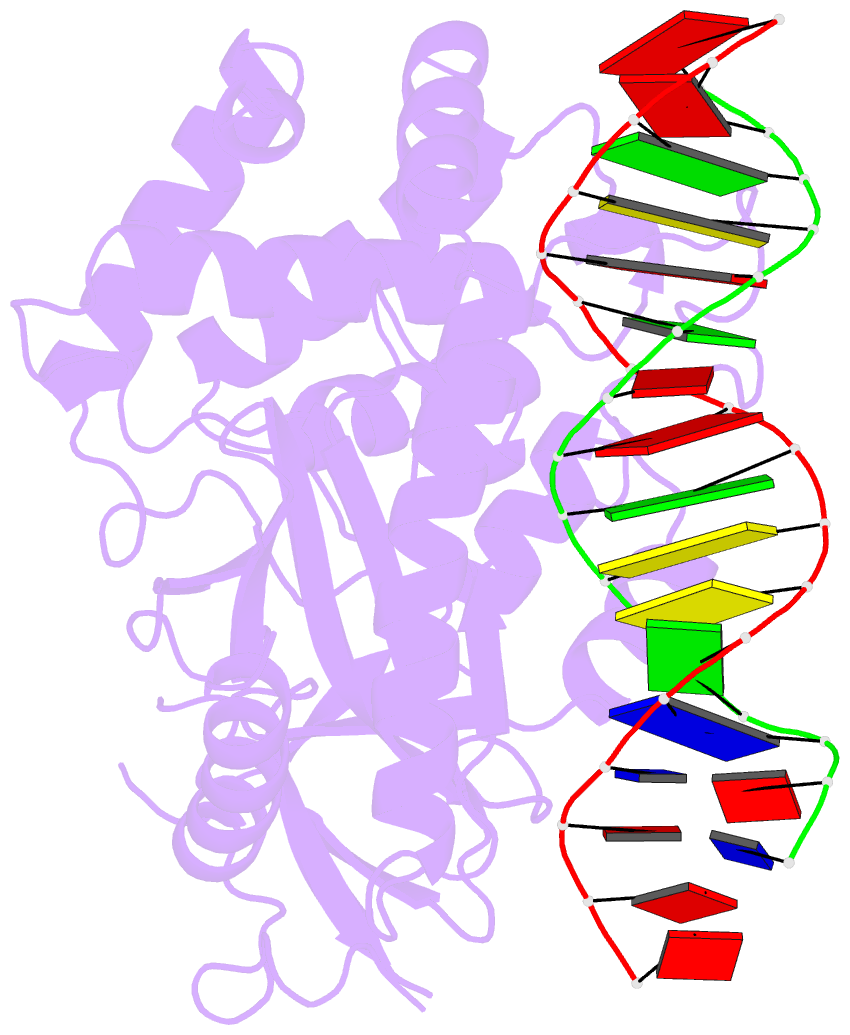
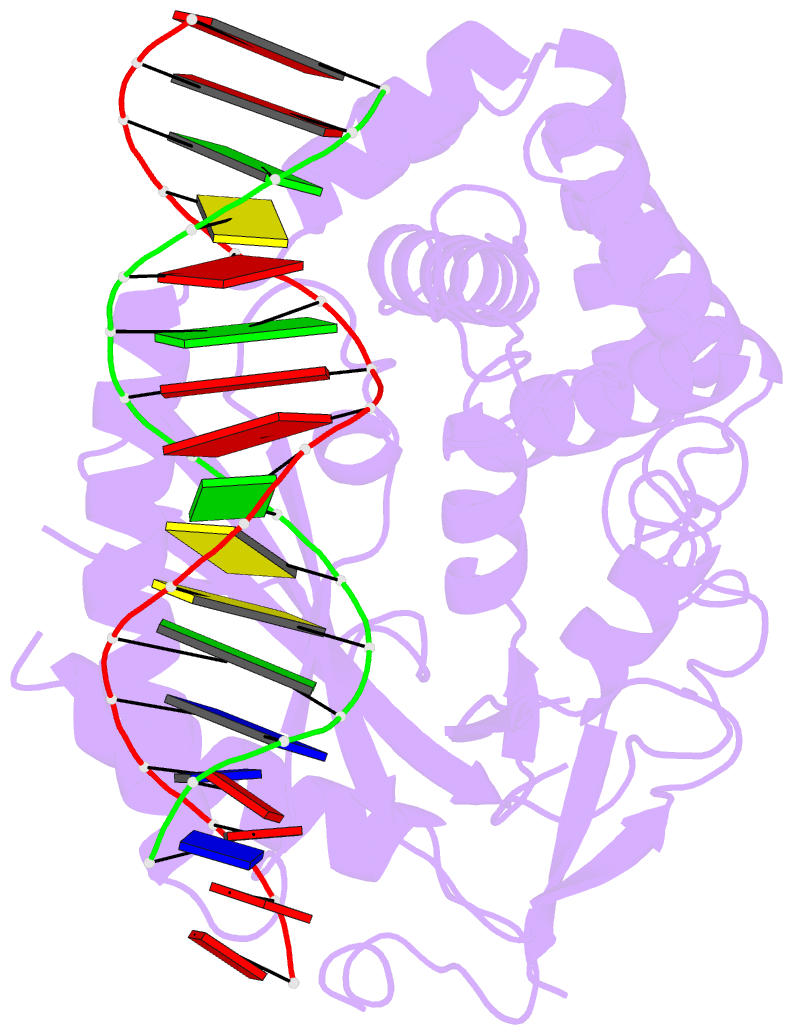
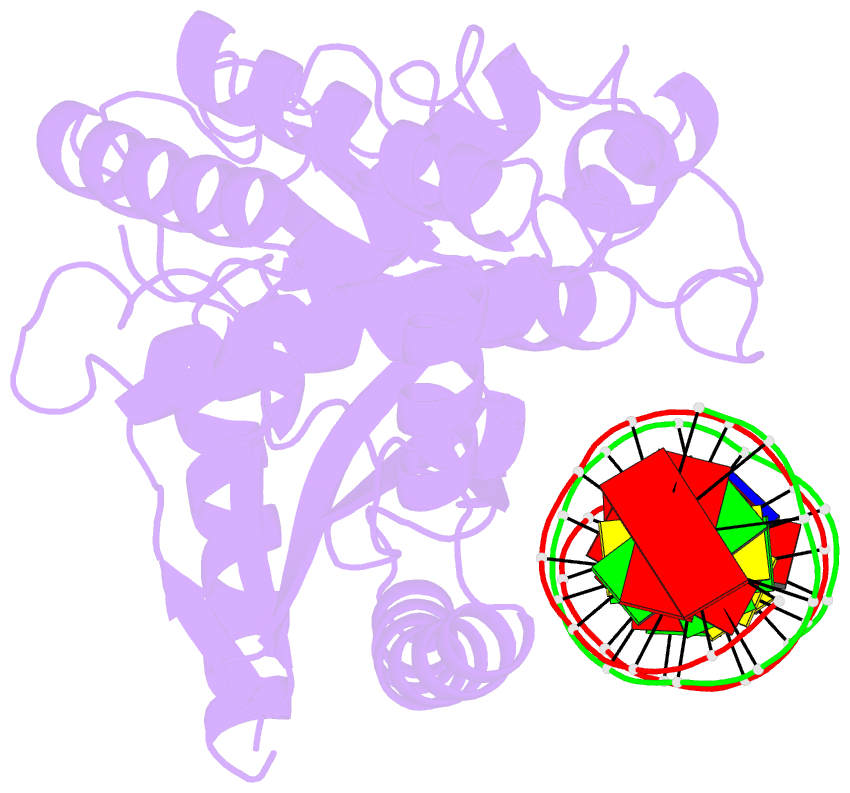
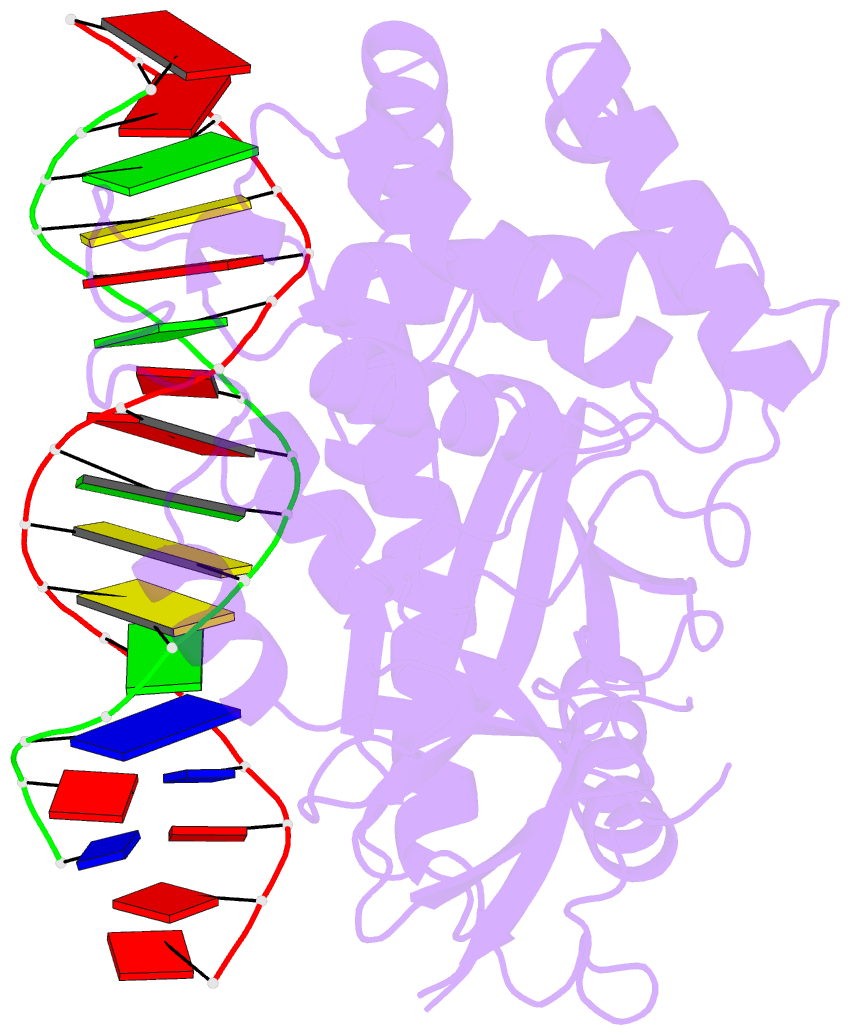
Summary information and primary citation
- PDB-id
- 6edc; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase-DNA
- Method
- X-ray (2.712 Å)
- Summary
- Hcgas-16bp dsDNA complex
- Reference
- Xie W, Lama L, Adura C, Tomita D, Glickman JF, Tuschl T, Patel DJ (2019): "Human cGAS catalytic domain has an additional DNA-binding interface that enhances enzymatic activity and liquid-phase condensation." Proc.Natl.Acad.Sci.USA, 116, 11946-11955. doi: 10.1073/pnas.1905013116.
- Abstract
- The cyclic GMP-AMP synthase (cGAS)-cGAMP-STING pathway plays a key role in innate immunity, with cGAS sensing both pathogenic and mislocalized DNA in the cytoplasm. Human cGAS (h-cGAS) constitutes an important drug target for control of antiinflammatory responses that can contribute to the onset of autoimmune diseases. Recent studies have established that the positively charged N-terminal segment of cGAS contributes to enhancement of cGAS enzymatic activity as a result of DNA-induced liquid-phase condensation. We have identified an additional cGASCD-DNA interface (labeled site-C; CD, catalytic domain) in the crystal structure of a human SRY.cGASCD-DNA complex, with mutations along this basic site-C cGAS interface disrupting liquid-phase condensation, as monitored by cGAMP formation, gel shift, spin-down, and turbidity assays, as well as time-lapse imaging of liquid droplet formation. We expand on an earlier ladder model of cGAS dimers bound to a pair of parallel-aligned DNAs to propose a multivalent interaction-mediated cluster model to account for DNA-mediated condensation involving both the N-terminal domain of cGAS and the site-C cGAS-DNA interface. We also report the crystal structure of the h-cGASCD-DNA complex containing a triple mutant that disrupts the site-C interface, with this complex serving as a future platform for guiding cGAS inhibitor development at the DNA-bound h-cGAS level. Finally, we solved the structure of RU.521 bound in two alternate alignments to apo h-cGASCD, thereby occupying more of the catalytic pocket and providing insights into further optimization of active-site-binding inhibitors.