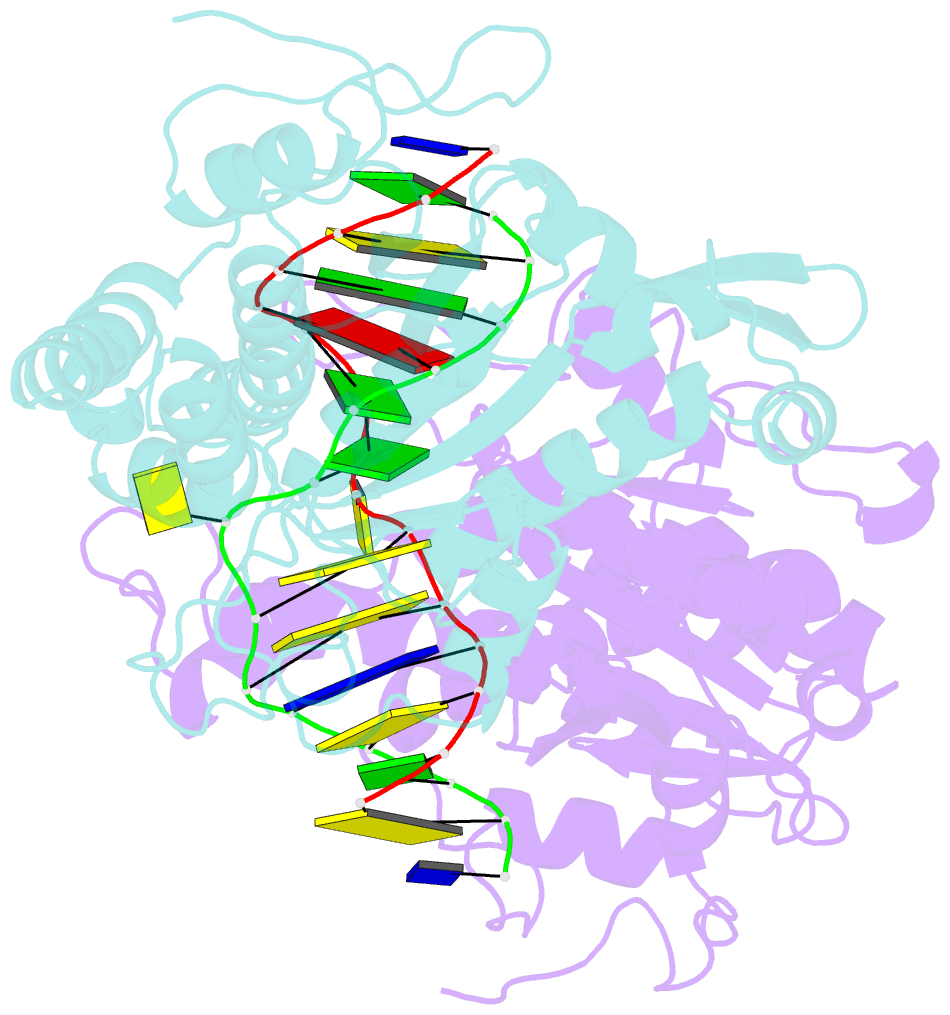
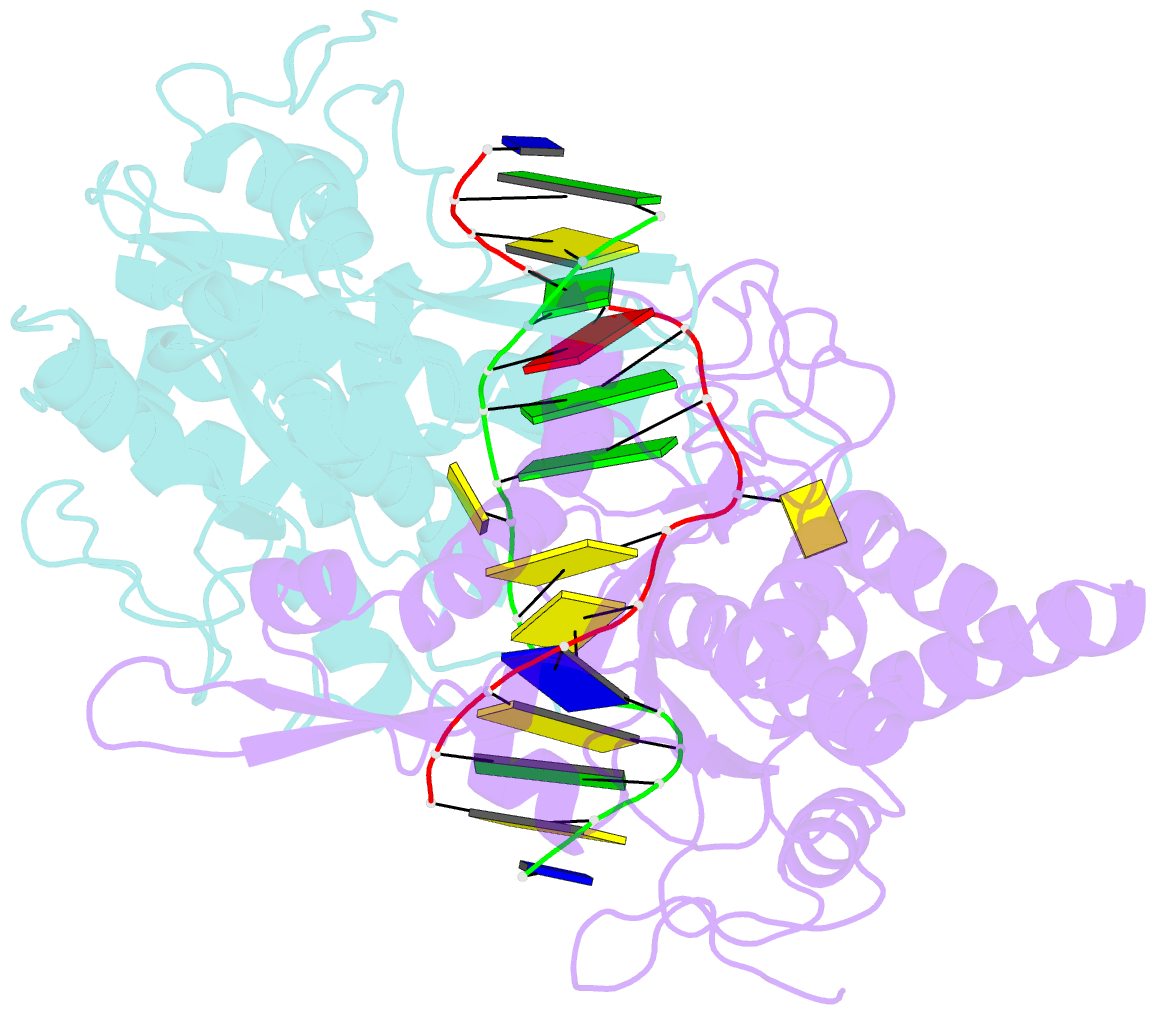
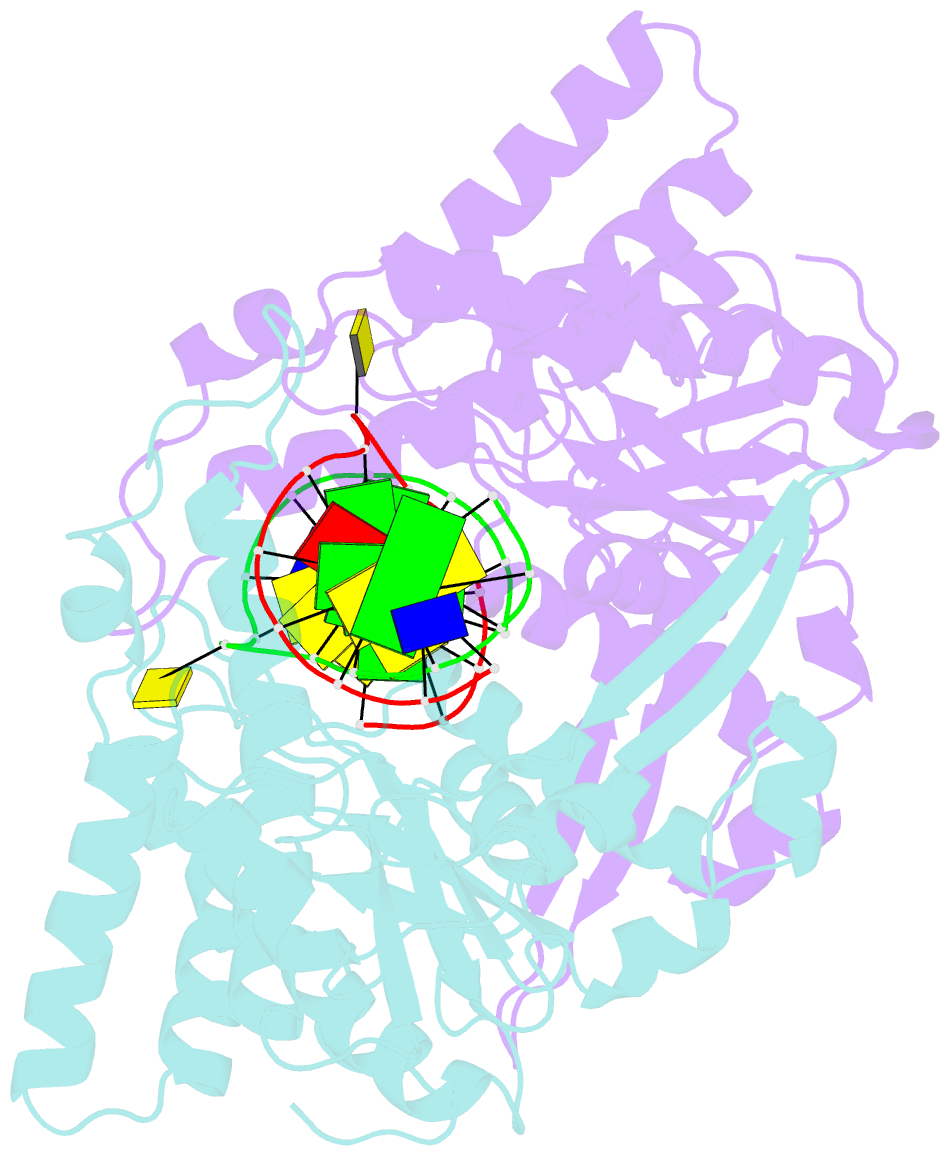
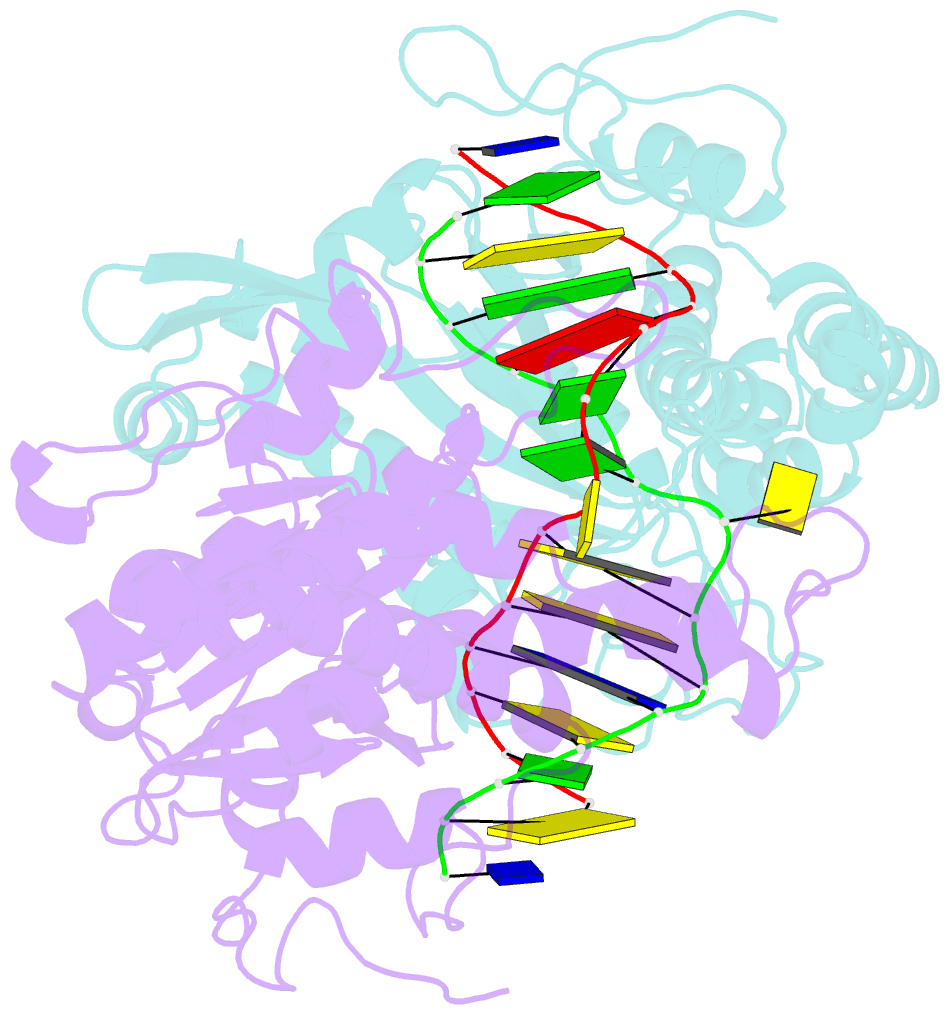
Summary information and primary citation
- PDB-id
- 6eko; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase
- Method
- X-ray (2.284 Å)
- Summary
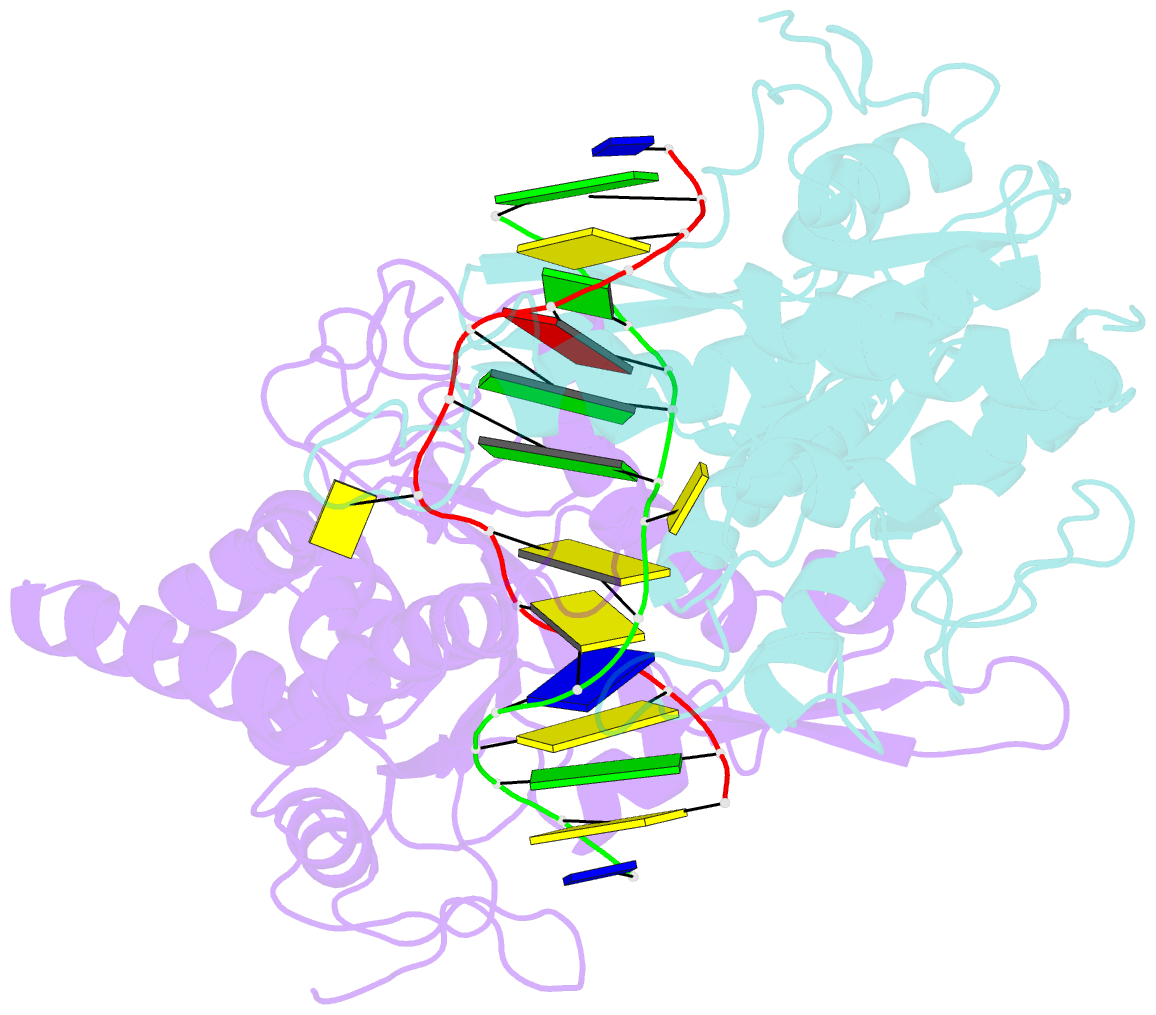
- Crystal structure of type iip restriction endonuclease pfoi with cognate DNA
- Reference
- Tamulaitiene G, Manakova E, Jovaisaite V, Tamulaitis G, Grazulis S, Bochtler M, Siksnys V (2019): "Unique mechanism of target recognition by PfoI restriction endonuclease of the CCGG-family." Nucleic Acids Res., 47, 997-1010. doi: 10.1093/nar/gky1137.
- Abstract
- Restriction endonucleases (REs) of the CCGG-family recognize a set of 4-8 bp target sequences that share a common CCGG or CCNGG core and possess PD…D/ExK nuclease fold. REs that interact with 5 bp sequence 5'-CCNGG flip the central N nucleotides and 'compress' the bound DNA to stack the inner base pairs to mimic the CCGG sequence. PfoI belongs to the CCGG-family and cleaves the 7 bp sequence 5'-T|CCNGGA ("|" designates cleavage position). We present here crystal structures of PfoI in free and DNA-bound forms that show unique active site arrangement and mechanism of sequence recognition. Structures and mutagenesis indicate that PfoI features a permuted E…ExD…K active site that differs from the consensus motif characteristic to other family members. Although PfoI also flips the central N nucleotides of the target sequence it does not 'compress' the bound DNA. Instead, PfoI induces a drastic change in DNA backbone conformation that shortens the distance between scissile phosphates to match that in the unperturbed CCGG sequence. Our data demonstrate the diversity and versatility of structural mechanisms employed by restriction enzymes for recognition of related DNA sequences.