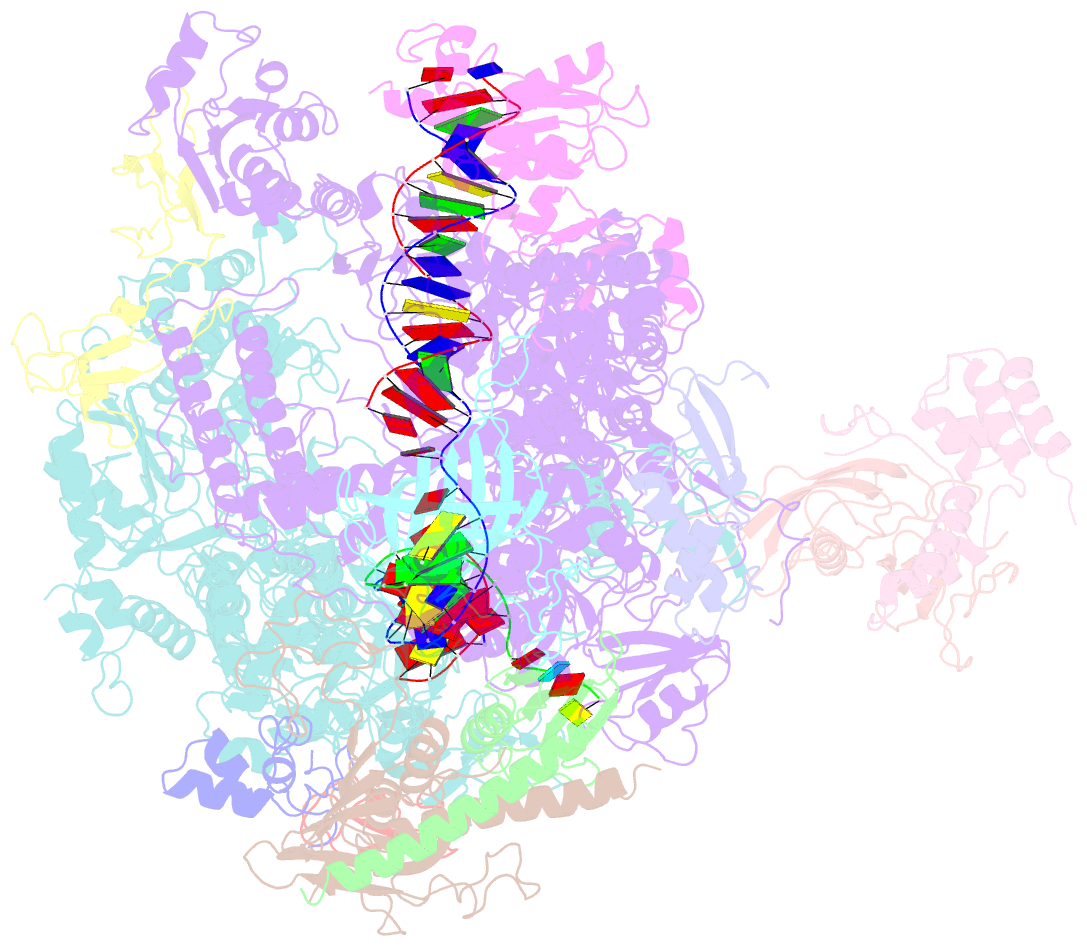
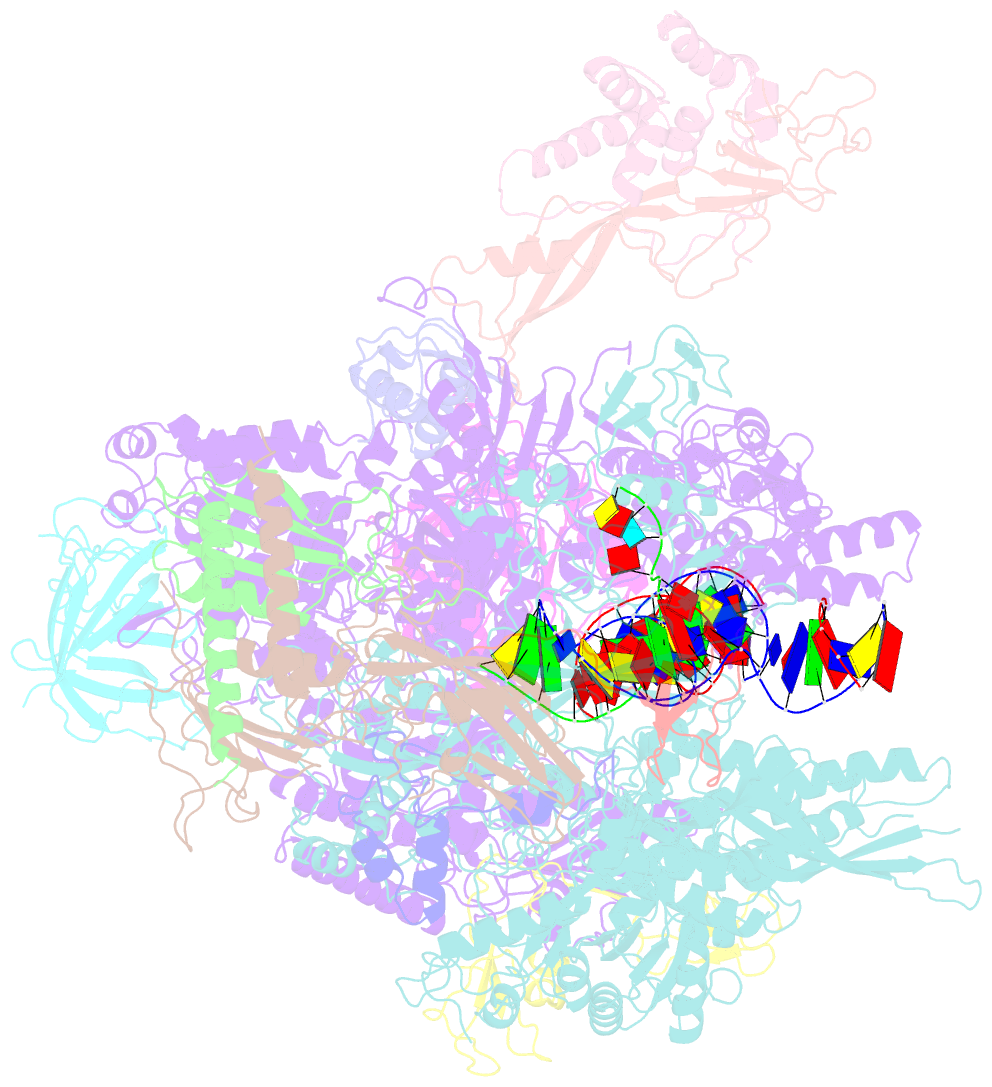
Summary information and primary citation
- PDB-id
- 6exv; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription
- Method
- cryo-EM (3.6 Å)
- Summary
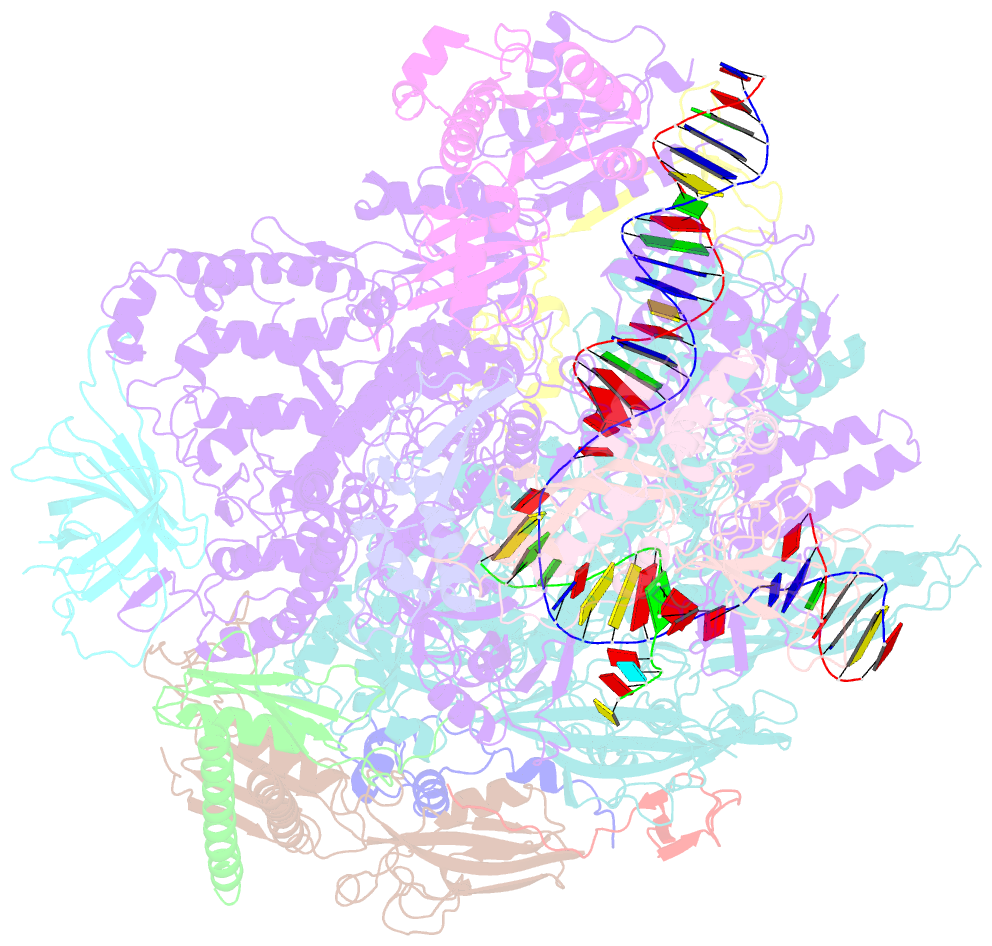
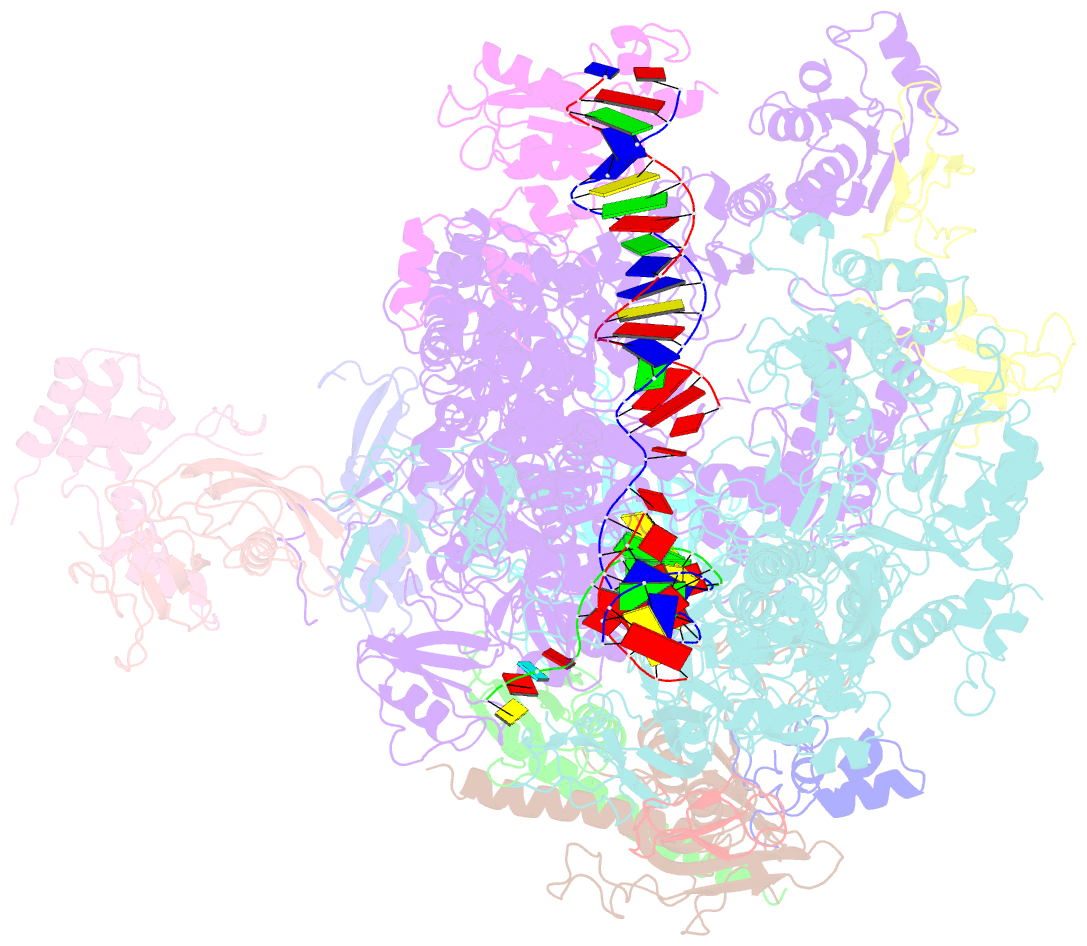
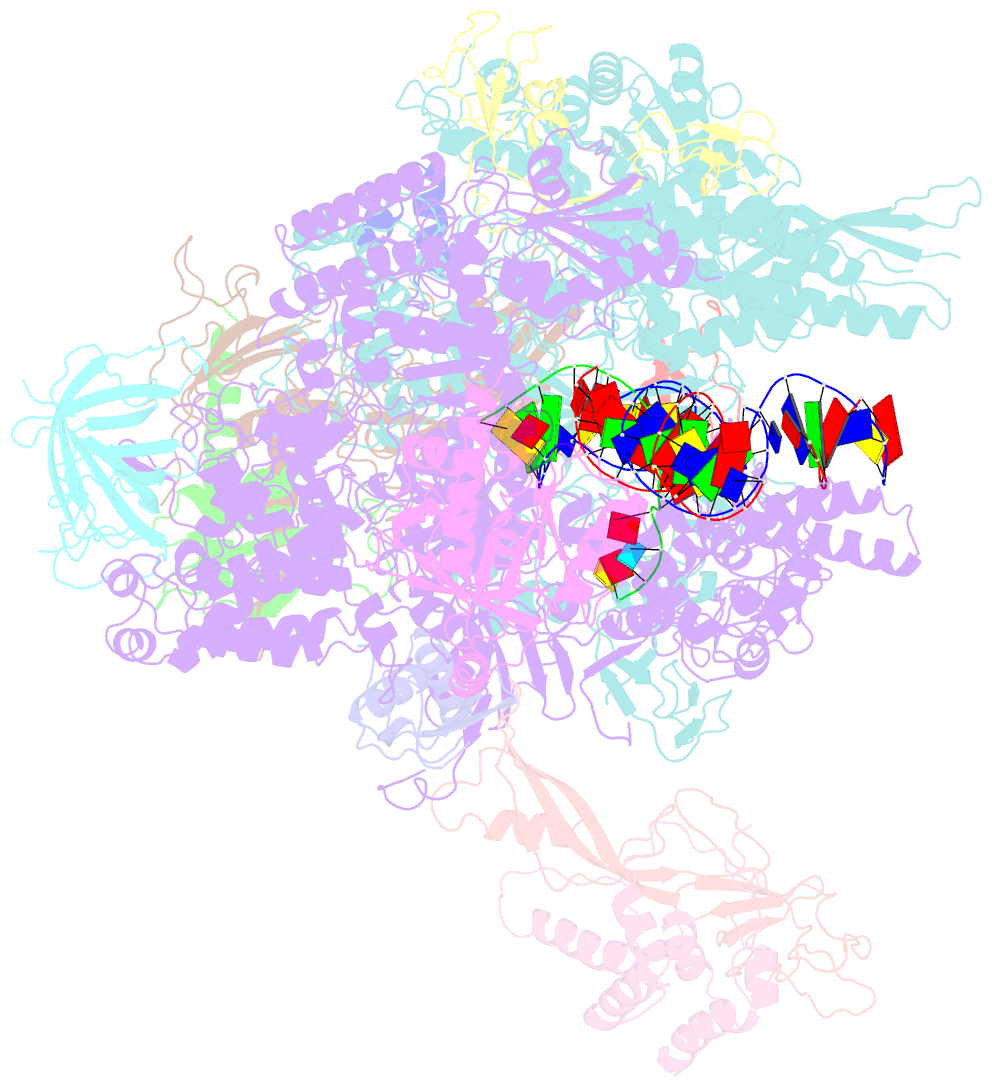
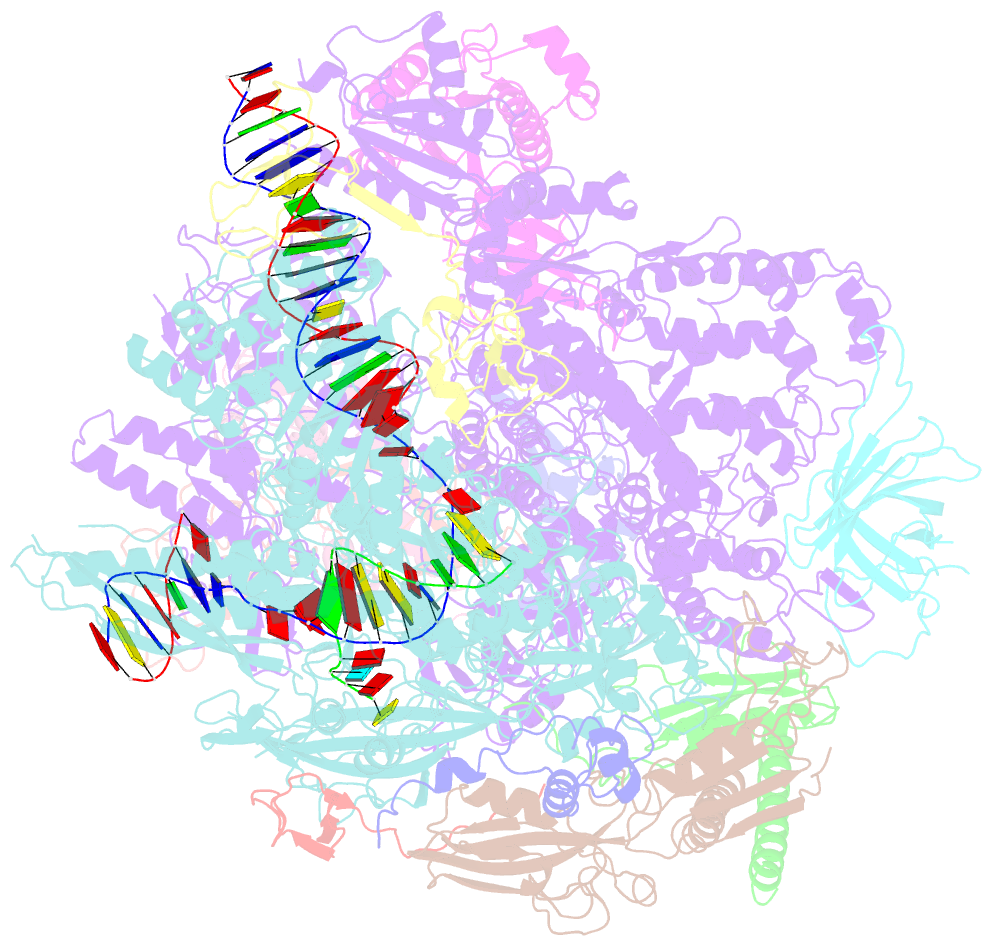
- Structure of mammalian RNA polymerase ii elongation complex inhibited by alpha-amanitin
- Reference
- Liu X, Farnung L, Wigge C, Cramer P (2018): "Cryo-EM structure of a mammalian RNA polymerase II elongation complex inhibited by alpha-amanitin." J. Biol. Chem., 293, 7189-7194. doi: 10.1074/jbc.RA118.002545.
- Abstract
- RNA polymerase II (Pol II) is the central enzyme that transcribes eukaryotic protein-coding genes to produce mRNA. The mushroom toxin α-amanitin binds Pol II and inhibits transcription at the step of RNA chain elongation. Pol II from yeast binds α-amanitin with micromolar affinity, whereas metazoan Pol II enzymes exhibit nanomolar affinities. Here, we present the high-resolution cryo-EM structure of α-amanitin bound to and inhibited by its natural target, the mammalian Pol II elongation complex. The structure revealed that the toxin is located in a pocket previously identified in yeast Pol II but forms additional contacts with metazoan-specific residues, which explains why its affinity to mammalian Pol II is ∼3000 times higher than for yeast Pol II. Our work provides the structural basis for the inhibition of mammalian Pol II by the natural toxin α-amanitin and highlights that cryo-EM is well suited to studying interactions of a small molecule with its macromolecular target.