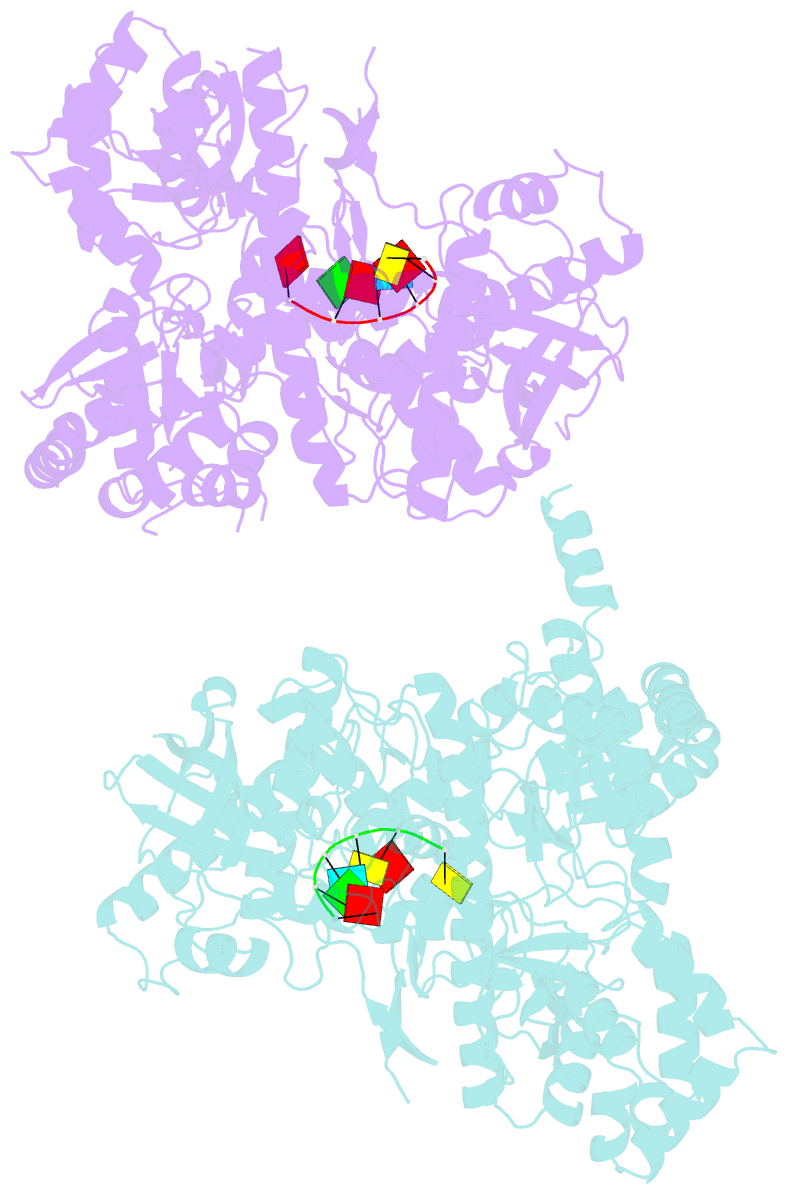
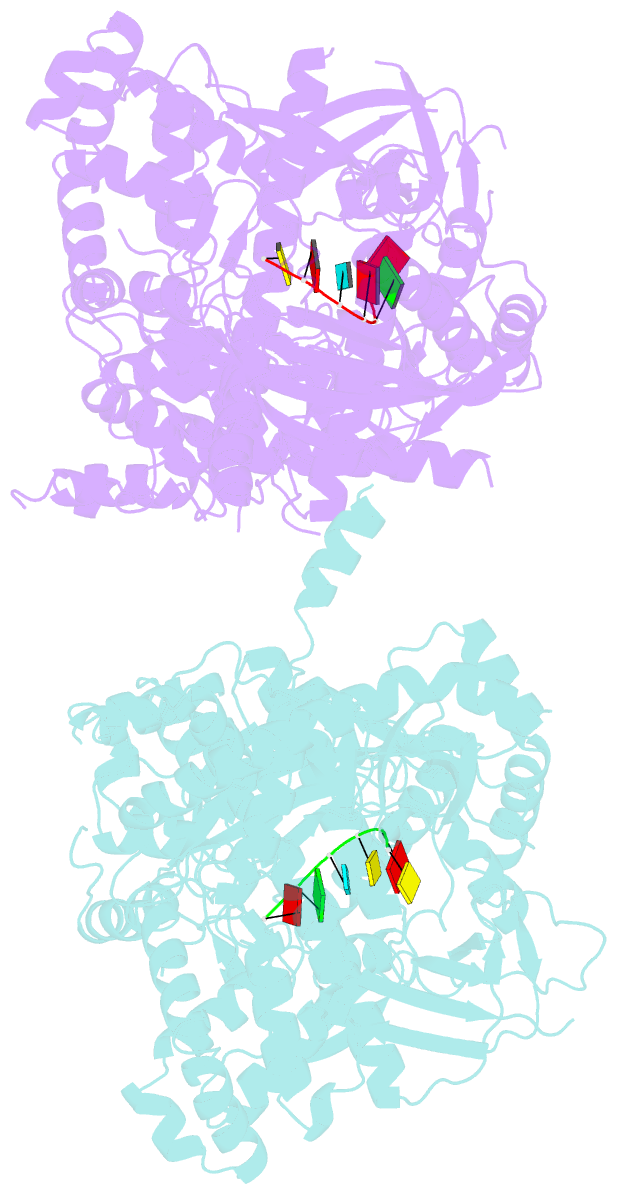
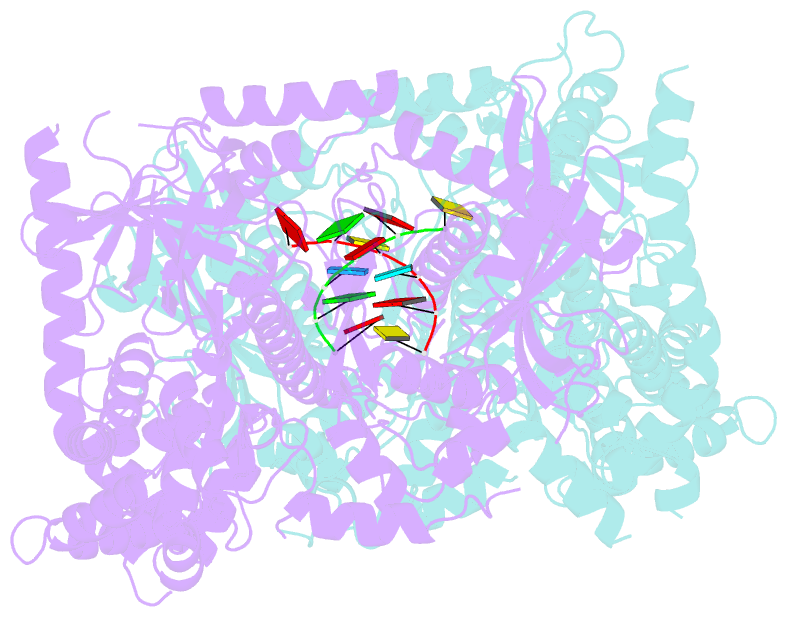
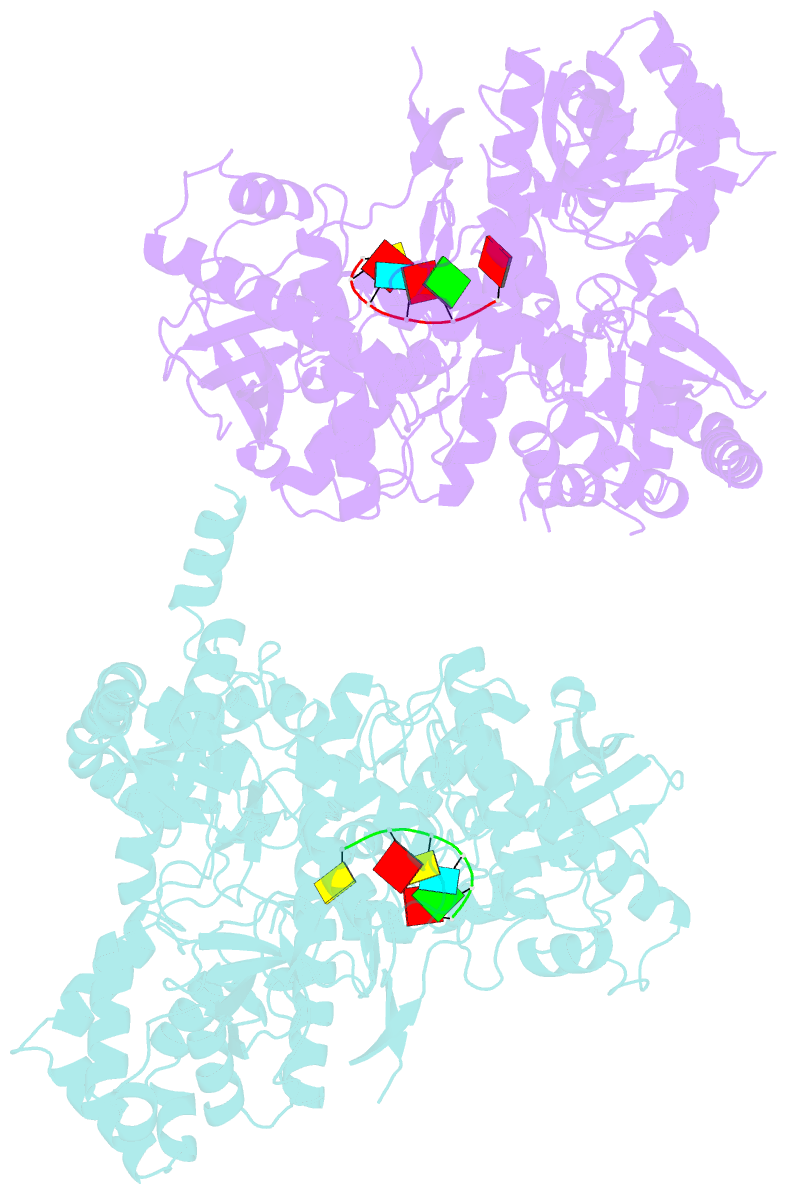
Summary information and primary citation
- PDB-id
-
6f3h;
DSSR-derived features in text and
JSON formats; DNAproDB
- Class
- hydrolase
- Method
- X-ray (2.703 Å)
- Summary
- Crystal structure of dss1 exoribonuclease active site
mutant d477n from candida glabrata
- Reference
-
Razew M, Warkocki Z, Taube M, Kolondra A,
Czarnocki-Cieciura M, Nowak E, Labedzka-Dmoch K, Kawinska
A, Piatkowski J, Golik P, Kozak M, Dziembowski A, Nowotny
M (2018): "Structural
analysis of mtEXO mitochondrial RNA degradosome reveals
tight coupling of nuclease and helicase components."
Nat Commun, 9, 97. doi:
10.1038/s41467-017-02570-5.
- Abstract
- Nuclease and helicase activities play pivotal roles in
various aspects of RNA processing and degradation. These
two activities are often present in multi-subunit complexes
from nucleic acid metabolism. In the mitochondrial
exoribonuclease complex (mtEXO) both enzymatic activities
are tightly coupled making it an excellent minimal system
to study helicase-exoribonuclease coordination. mtEXO is
composed of Dss1 3'-to-5' exoribonuclease and Suv3
helicase. It is the master regulator of mitochondrial gene
expression in yeast. Here, we present the structure of
mtEXO and a description of its mechanism of action. The
crystal structure of Dss1 reveals domains that are
responsible for interactions with Suv3. Importantly, these
interactions are compatible with the conformational changes
of Suv3 domains during the helicase cycle. We demonstrate
that mtEXO is an intimate complex which forms an
RNA-binding channel spanning its entire structure, with
Suv3 helicase feeding the 3' end of the RNA toward the
active site of Dss1.