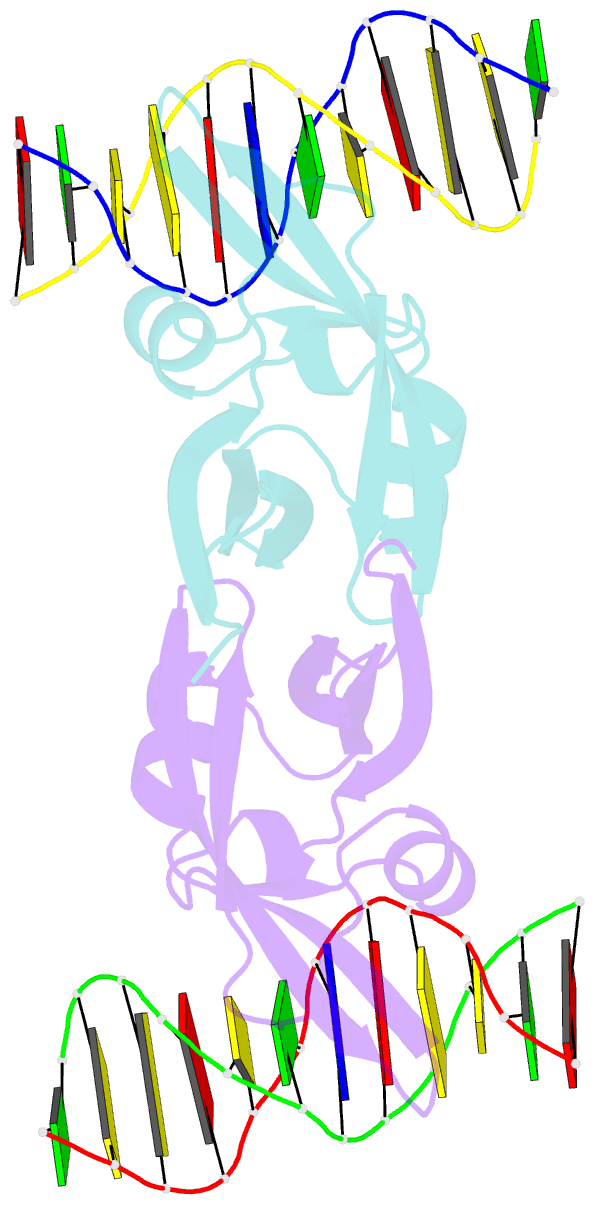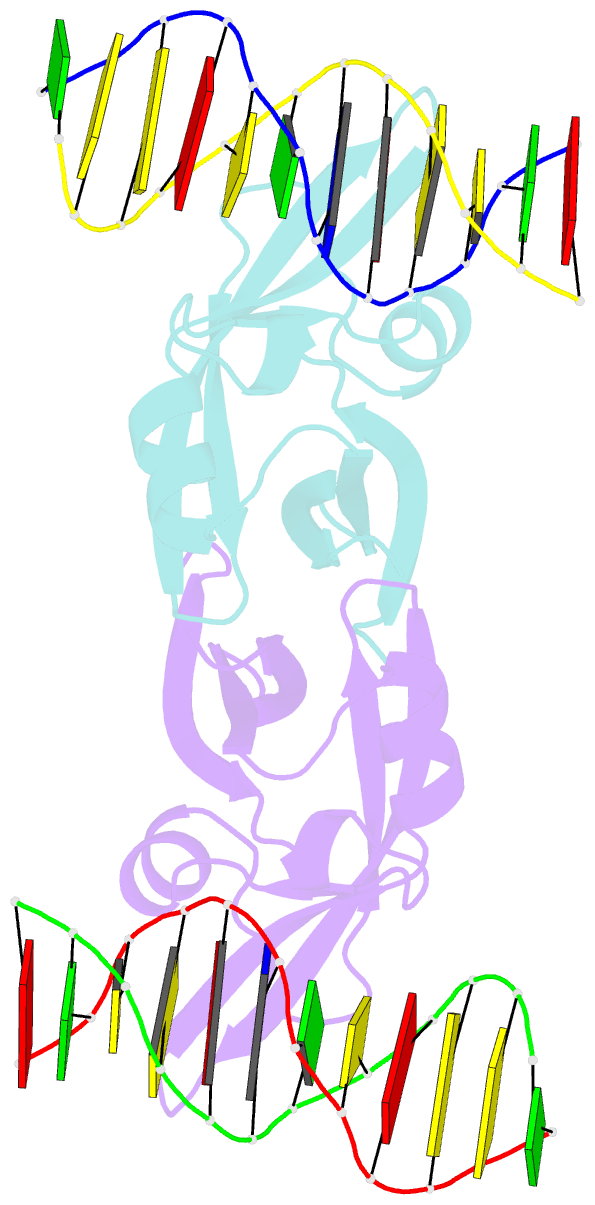Summary information and primary citation
- PDB-id
- 6fas; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein
- Method
- X-ray (1.9 Å)
- Summary
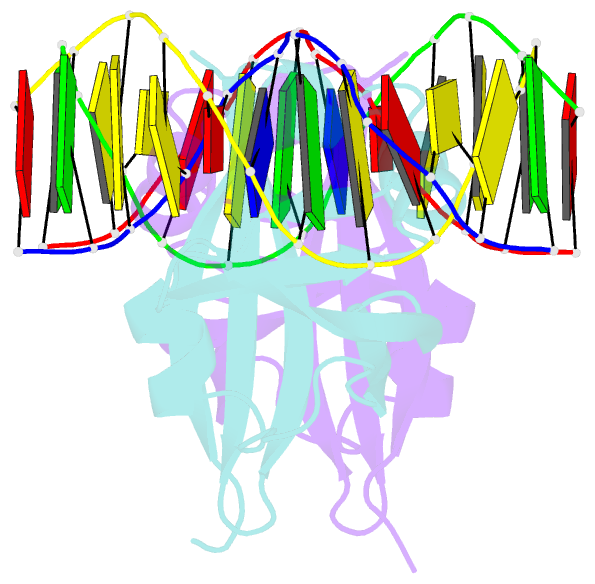
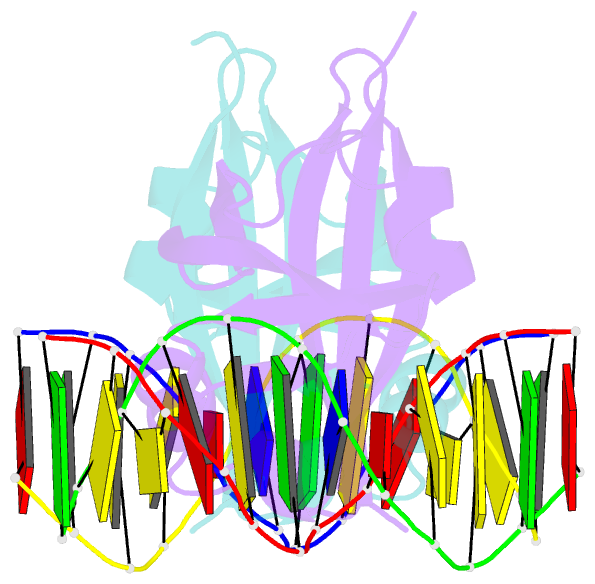
- Crystal structure of val1 b3 domain in complex with cognate DNA
- Reference
- Sasnauskas G, Kauneckaite K, Siksnys V (2018): "Structural basis of DNA target recognition by the B3 domain of Arabidopsis epigenome reader VAL1." Nucleic Acids Res., 46, 4316-4324. doi: 10.1093/nar/gky256.
- Abstract
- Arabidopsis thaliana requires a prolonged period of cold exposure during winter to initiate flowering in a process termed vernalization. Exposure to cold induces epigenetic silencing of the FLOWERING LOCUS C (FLC) gene by Polycomb group (PcG) proteins. A key role in this epigenetic switch is played by transcriptional repressors VAL1 and VAL2, which specifically recognize Sph/RY DNA sequences within FLC via B3 DNA binding domains, and mediate recruitment of PcG silencing machinery. To understand the structural mechanism of site-specific DNA recognition by VAL1, we have solved the crystal structure of VAL1 B3 domain (VAL1-B3) bound to a 12 bp oligoduplex containing the canonical Sph/RY DNA sequence 5'-CATGCA-3'/5'-TGCATG-3'. We find that VAL1-B3 makes H-bonds and van der Waals contacts to DNA bases of all six positions of the canonical Sph/RY element. In agreement with the structure, in vitro DNA binding studies show that VAL1-B3 does not tolerate substitutions at any position of the 5'-TGCATG-3' sequence. The VAL1-B3-DNA structure presented here provides a structural model for understanding the specificity of plant B3 domains interacting with the Sph/RY and other DNA sequences.