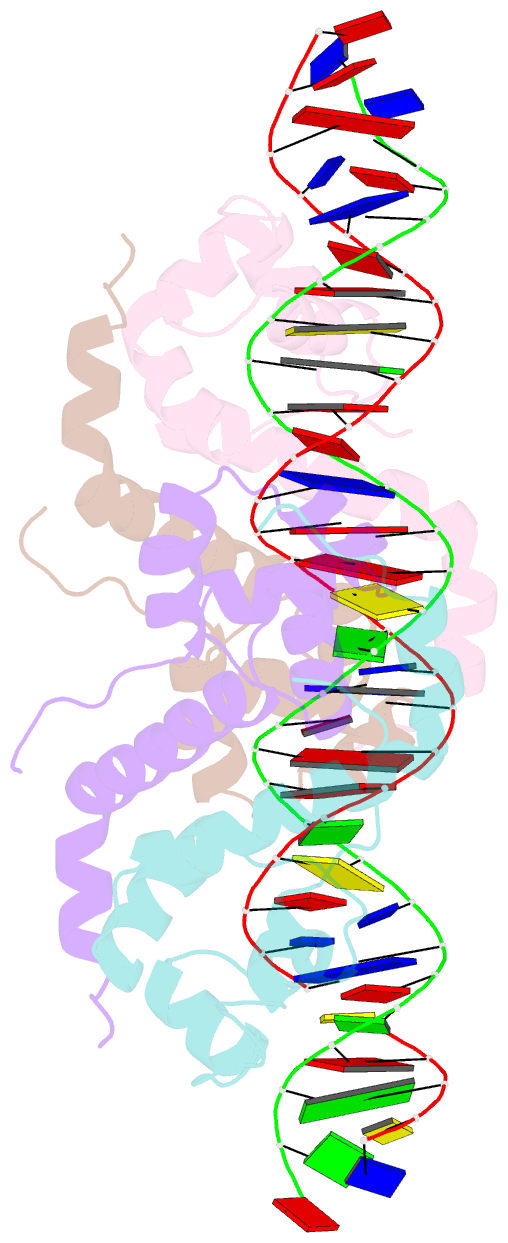
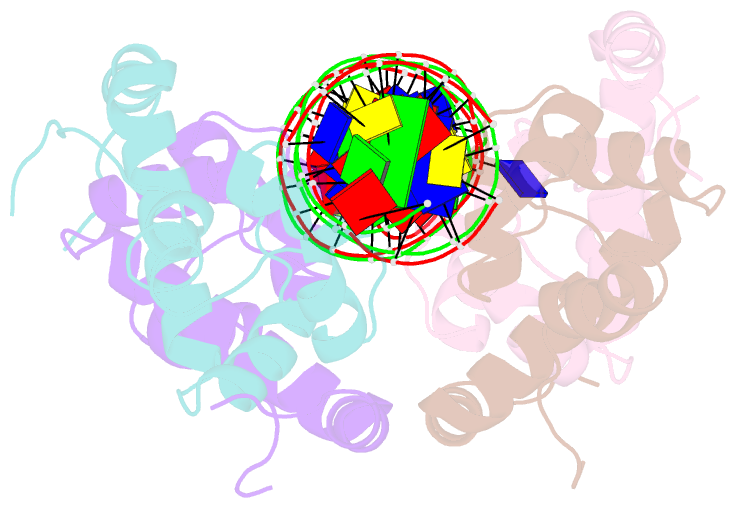
Summary information and primary citation
- PDB-id
- 6fix; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- antitoxin
- Method
- X-ray (3.8 Å)
- Summary
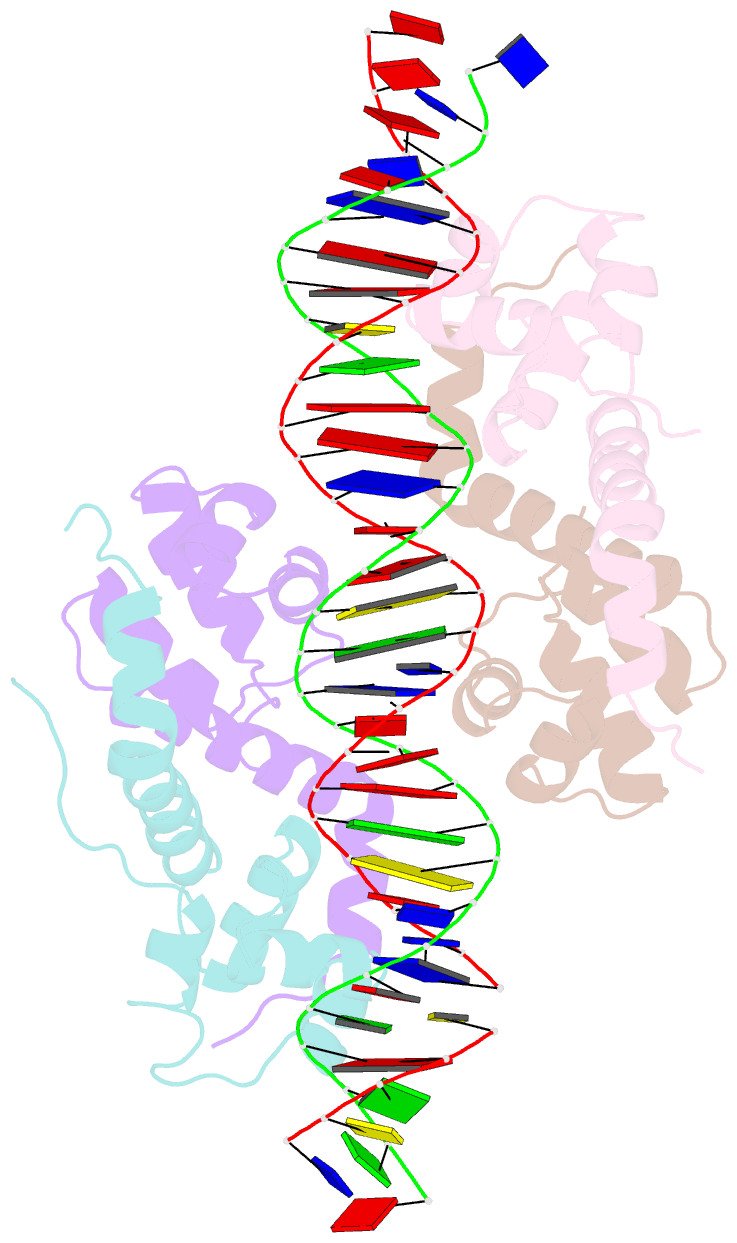
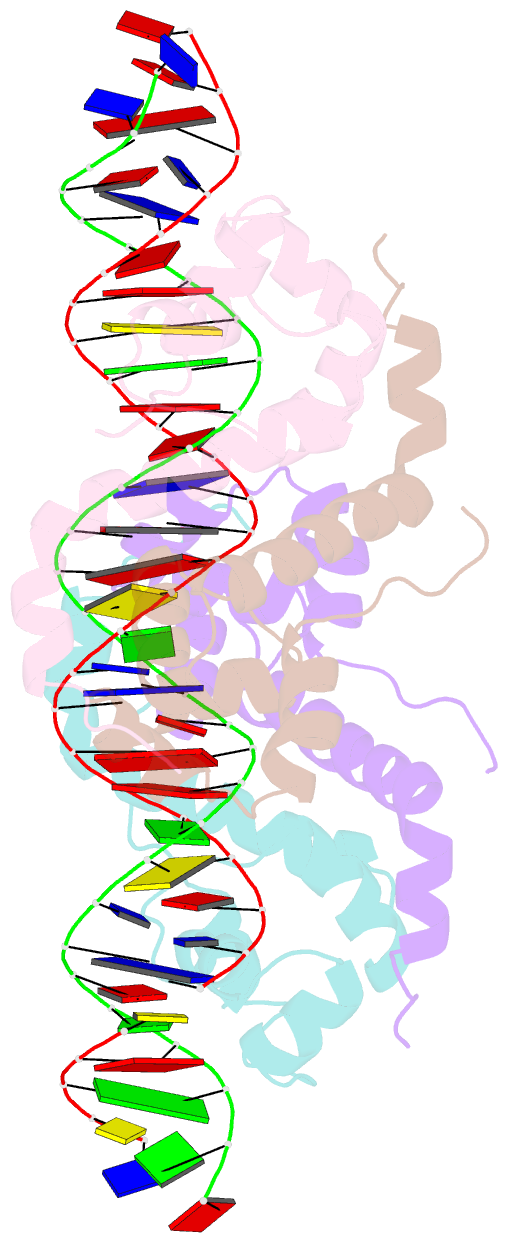
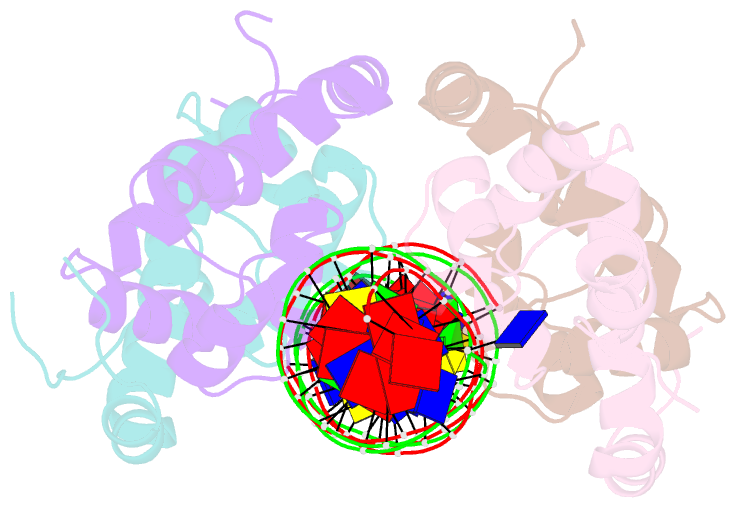
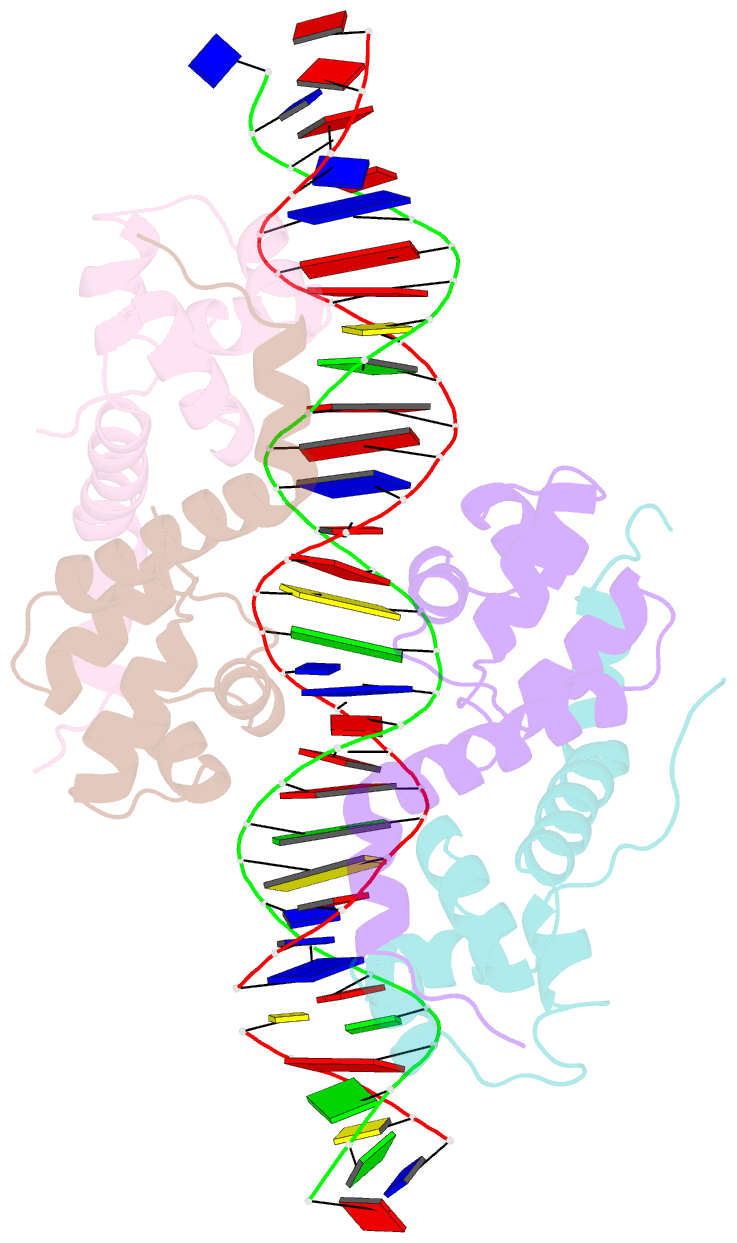
- Antitoxin graa in complex with its operator
- Reference
- Talavera A, Tamman H, Ainelo A, Konijnenberg A, Hadzi S, Sobott F, Garcia-Pino A, Horak R, Loris R (2019): "A dual role in regulation and toxicity for the disordered N-terminus of the toxin GraT." Nat Commun, 10, 972. doi: 10.1038/s41467-019-08865-z.
- Abstract
- Bacterial toxin-antitoxin (TA) modules are tightly regulated to maintain growth in favorable conditions or growth arrest during stress. A typical regulatory strategy involves the antitoxin binding and repressing its own promoter while the toxin often acts as a co-repressor. Here we show that Pseudomonas putida graTA-encoded antitoxin GraA and toxin GraT differ from other TA proteins in the sense that not the antitoxin but the toxin possesses a flexible region. GraA auto-represses the graTA promoter: two GraA dimers bind cooperatively at opposite sides of the operator sequence. Contrary to other TA modules, GraT is a de-repressor of the graTA promoter as its N-terminal disordered segment prevents the binding of the GraT2A2 complex to the operator. Removal of this region restores operator binding and abrogates Gr aT toxicity. GraTA represents a TA module where a flexible region in the toxin rather than in the antitoxin controls operon expression and toxin activity.