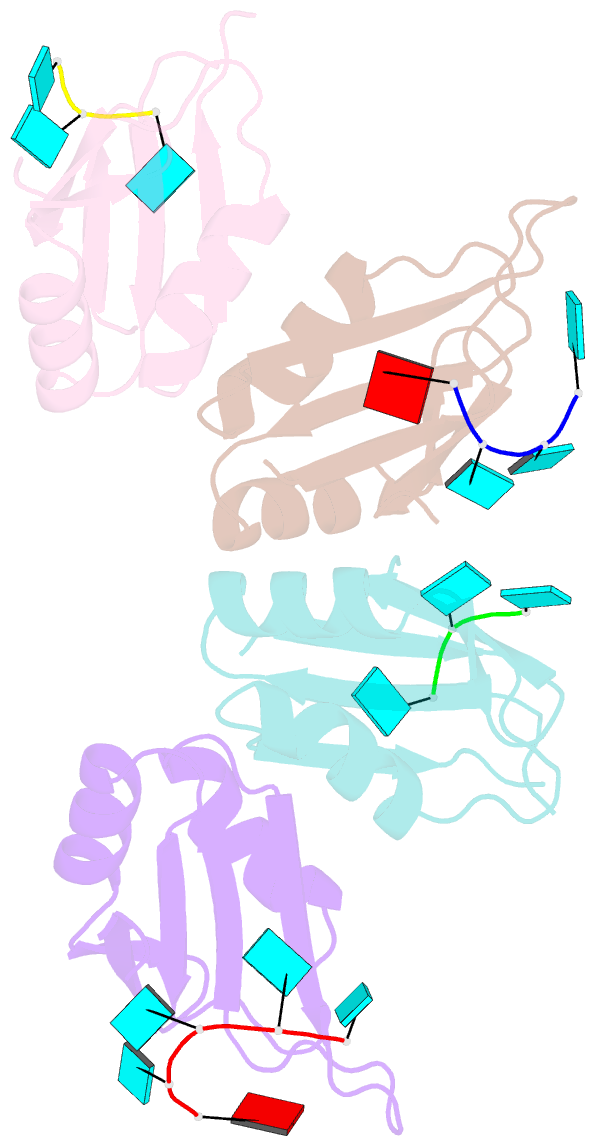
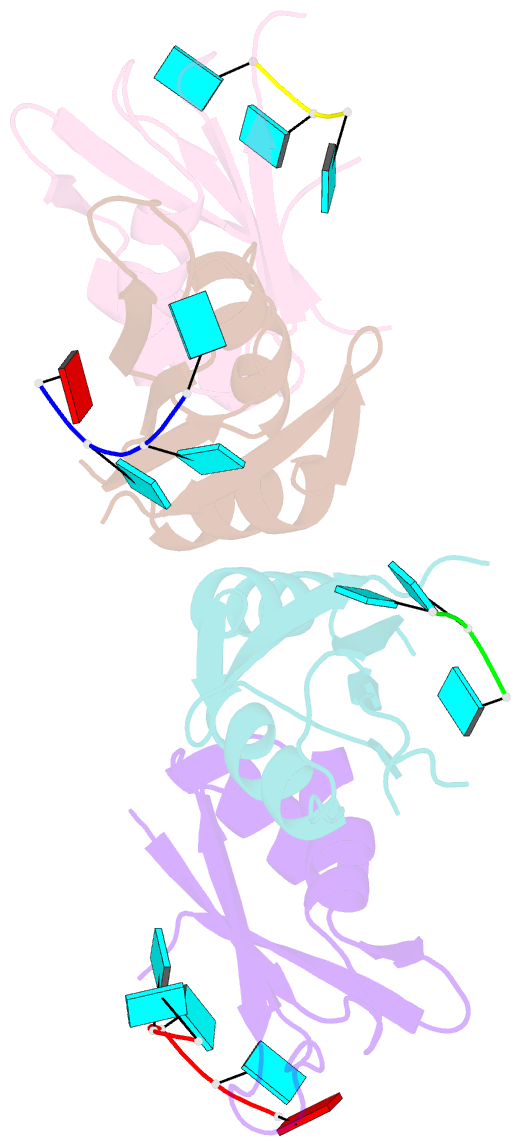
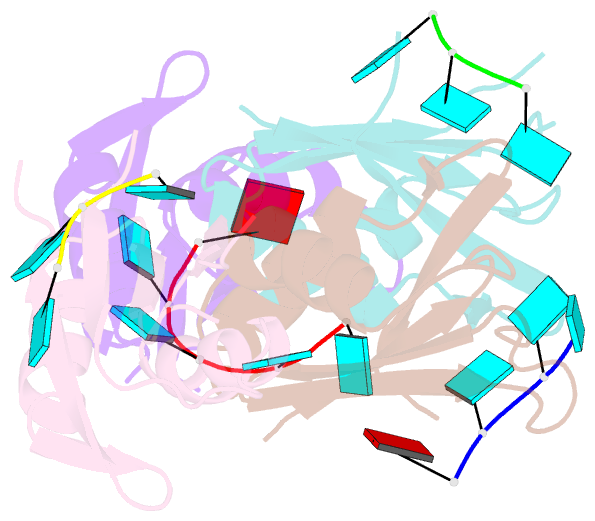
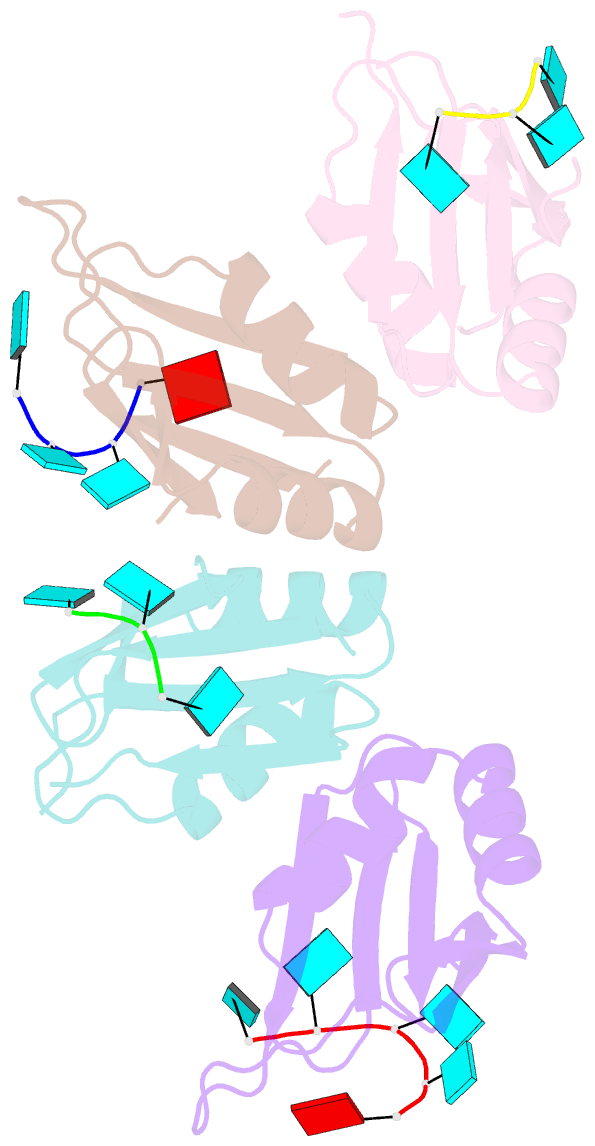
Summary information and primary citation
- PDB-id
- 6gc5; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein
- Method
- X-ray (1.9 Å)
- Summary
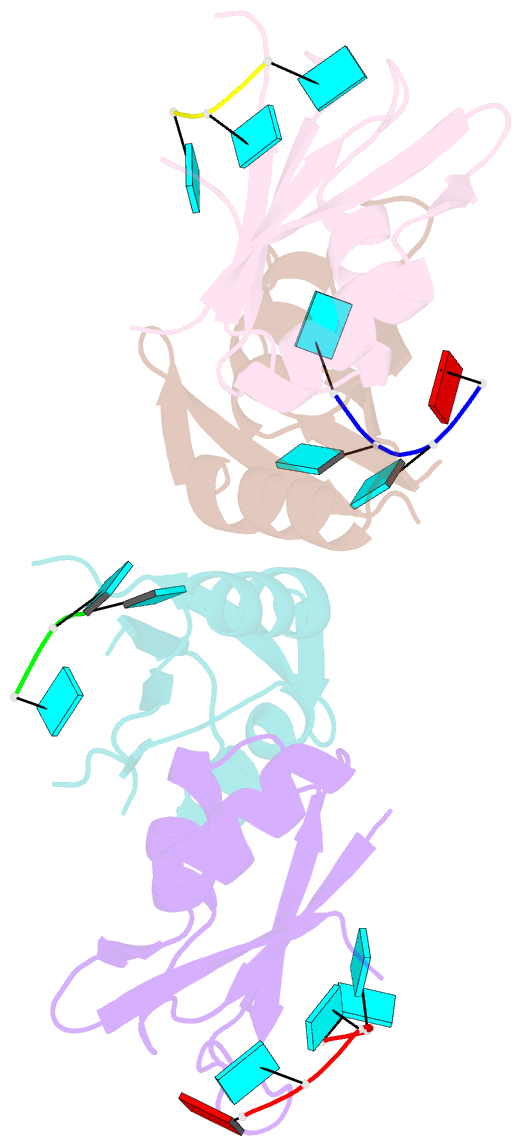
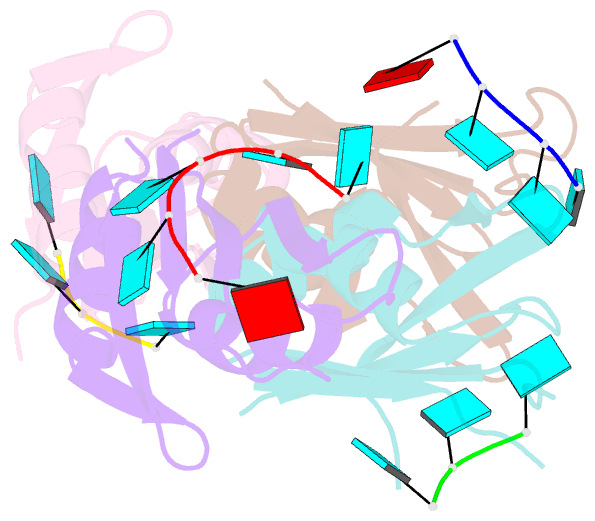
- Molecular basis for au-rich element recognition and dimerization by the hur c-terminal rrm
- Reference
- Ripin N, Boudet J, Duszczyk MM, Hinniger A, Faller M, Krepl M, Gadi A, Schneider RJ, Sponer J, Meisner-Kober NC, Allain FH (2019): "Molecular basis for AU-rich element recognition and dimerization by the HuR C-terminal RRM." Proc. Natl. Acad. Sci. U.S.A., 116, 2935-2944. doi: 10.1073/pnas.1808696116.
- Abstract
- Human antigen R (HuR) is a key regulator of cellular mRNAs containing adenylate/uridylate-rich elements (AU-rich elements; AREs). These are a major class of cis elements within 3' untranslated regions, targeting these mRNAs for rapid degradation. HuR contains three RNA recognition motifs (RRMs): a tandem RRM1 and 2, followed by a flexible linker and a C-terminal RRM3. While RRM1 and 2 are structurally characterized, little is known about RRM3. Here we present a 1.9-Å-resolution crystal structure of RRM3 bound to different ARE motifs. This structure together with biophysical methods and cell-culture assays revealed the mechanism of RRM3 ARE recognition and dimerization. While multiple RNA motifs can be bound, recognition of the canonical AUUUA pentameric motif is possible by binding to two registers. Additionally, RRM3 forms homodimers to increase its RNA binding affinity. Finally, although HuR stabilizes ARE-containing RNAs, we found that RRM3 counteracts this effect, as shown in a cell-based ARE reporter assay and by qPCR with native HuR mRNA targets containing multiple AUUUA motifs, possibly by competing with RRM12.