Summary information and primary citation
- PDB-id
- 6gpg; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- antiviral protein
- Method
- X-ray (2.894 Å)
- Summary
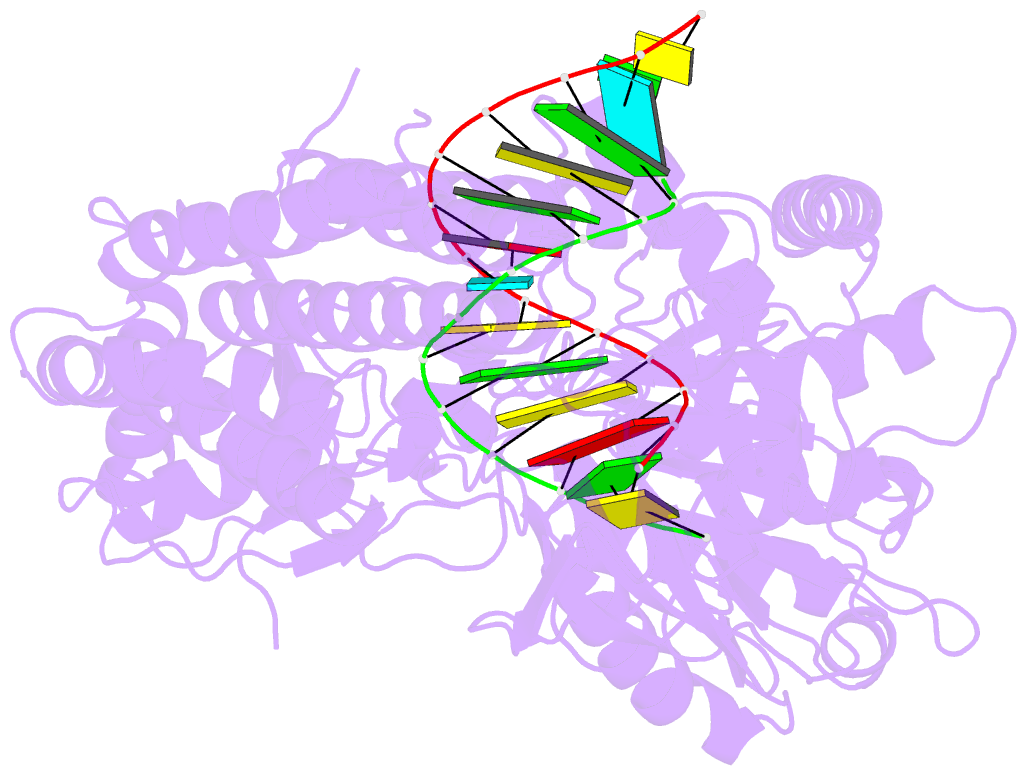
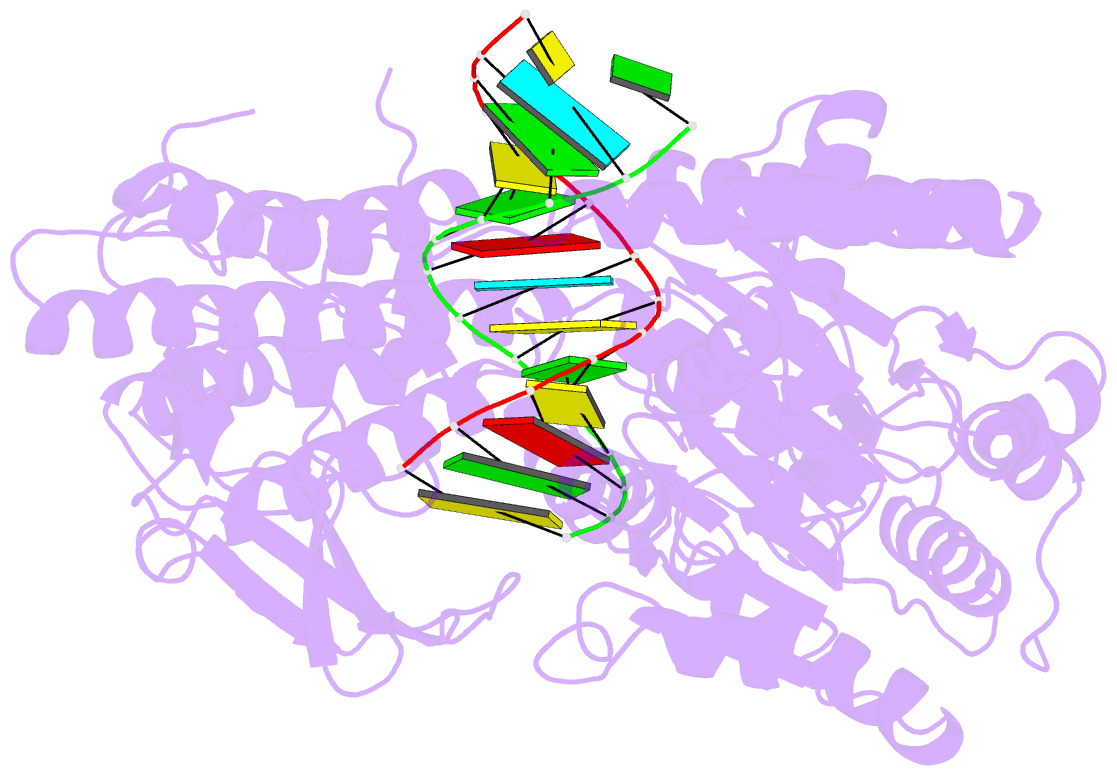
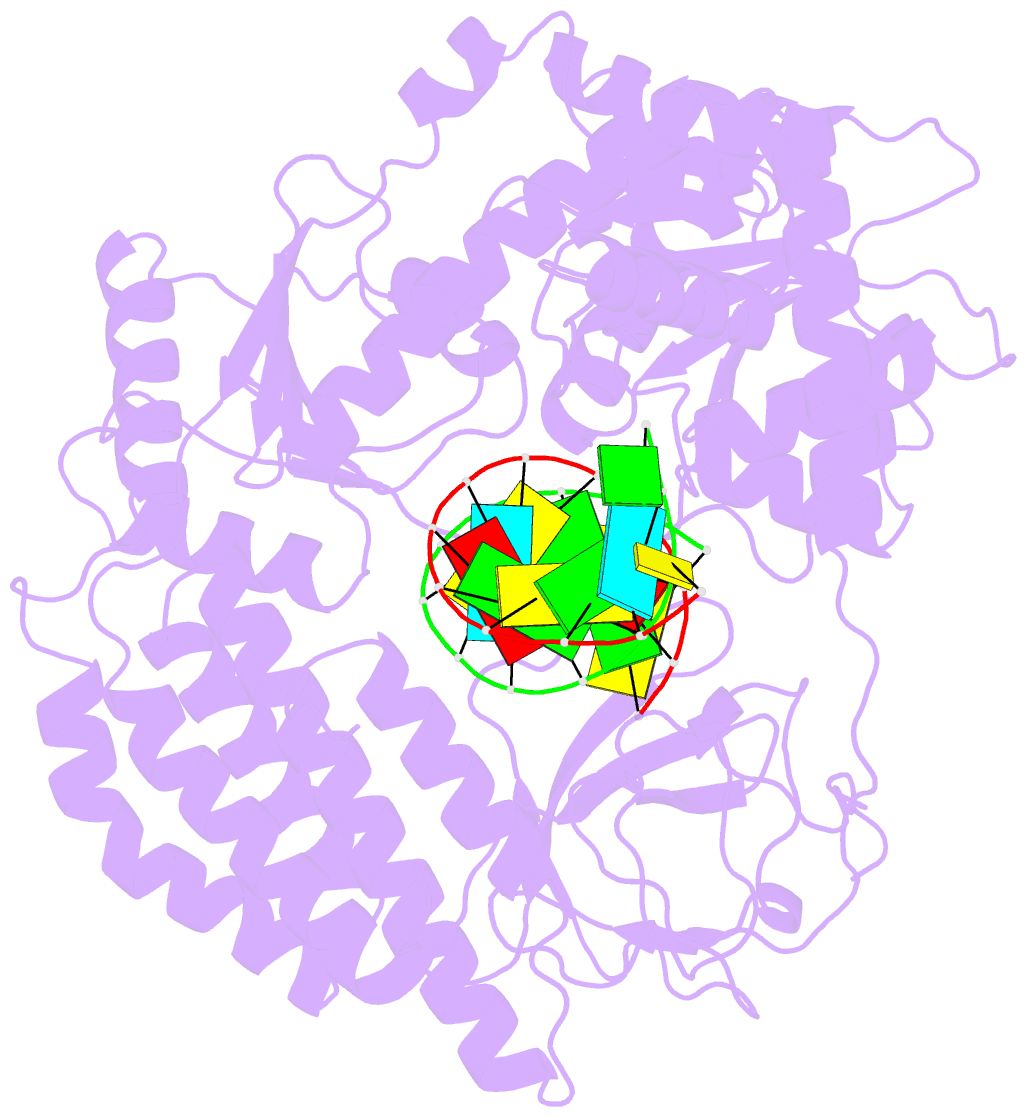
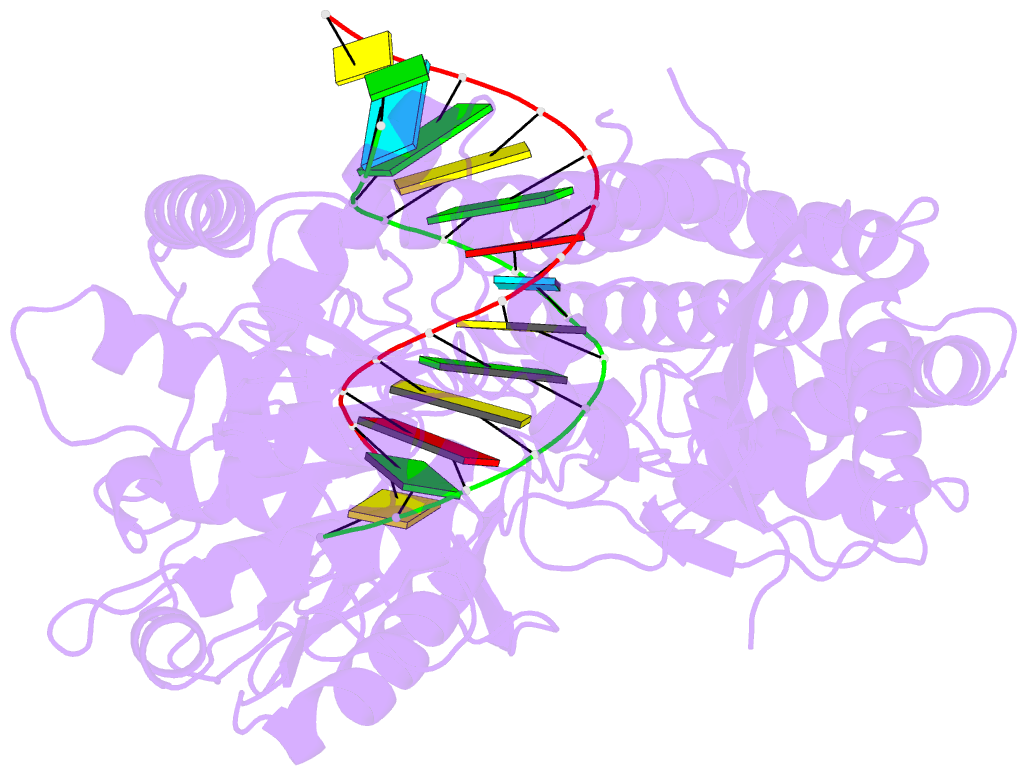
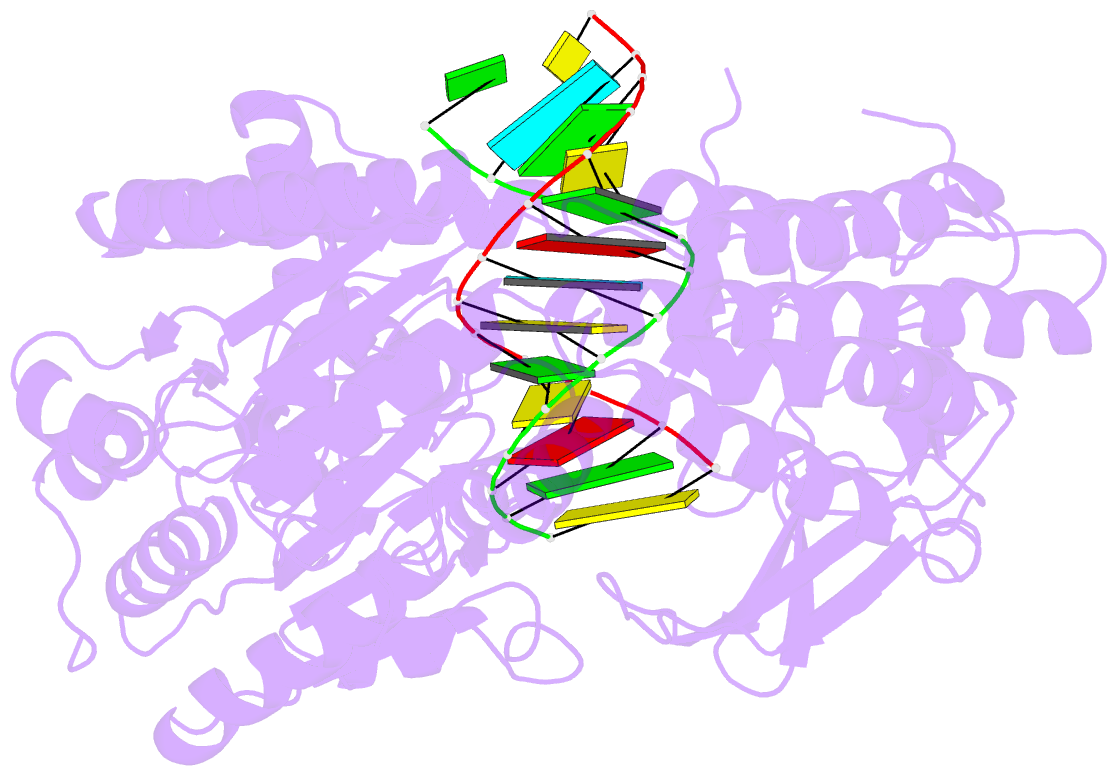
- Structure of the rig-i singleton-merten syndrome variant c268f
- Reference
- Lassig C, Lammens K, Gorenflos Lopez JL, Michalski S, Fettscher O, Hopfner KP (2018): "Unified mechanisms for self-RNA recognition by RIG-I Singleton-Merten syndrome variants." Elife, 7. doi: 10.7554/eLife.38958.
- Abstract
- The innate immune sensor retinoic acid-inducible gene I (RIG-I) detects cytosolic viral RNA and requires a conformational change caused by both ATP and RNA binding to induce an active signaling state and to trigger an immune response. Previously, we showed that ATP hydrolysis removes RIG-I from lower-affinity self-RNAs (Lässig et al., 2015), revealing how ATP turnover helps RIG-I distinguish viral from self-RNA and explaining why a mutation in a motif that slows down ATP hydrolysis causes the autoimmune disease Singleton-Merten syndrome (SMS). Here we show that a different, mechanistically unexplained SMS variant, C268F, which is localized in the ATP-binding P-loop, can signal independently of ATP but is still dependent on RNA. The structure of RIG-I C268F in complex with double-stranded RNA reveals that C268F helps induce a structural conformation in RIG-I that is similar to that induced by ATP. Our results uncover an unexpected mechanism to explain how a mutation in a P-loop ATPase can induce a gain-of-function ATP state in the absence of ATP.