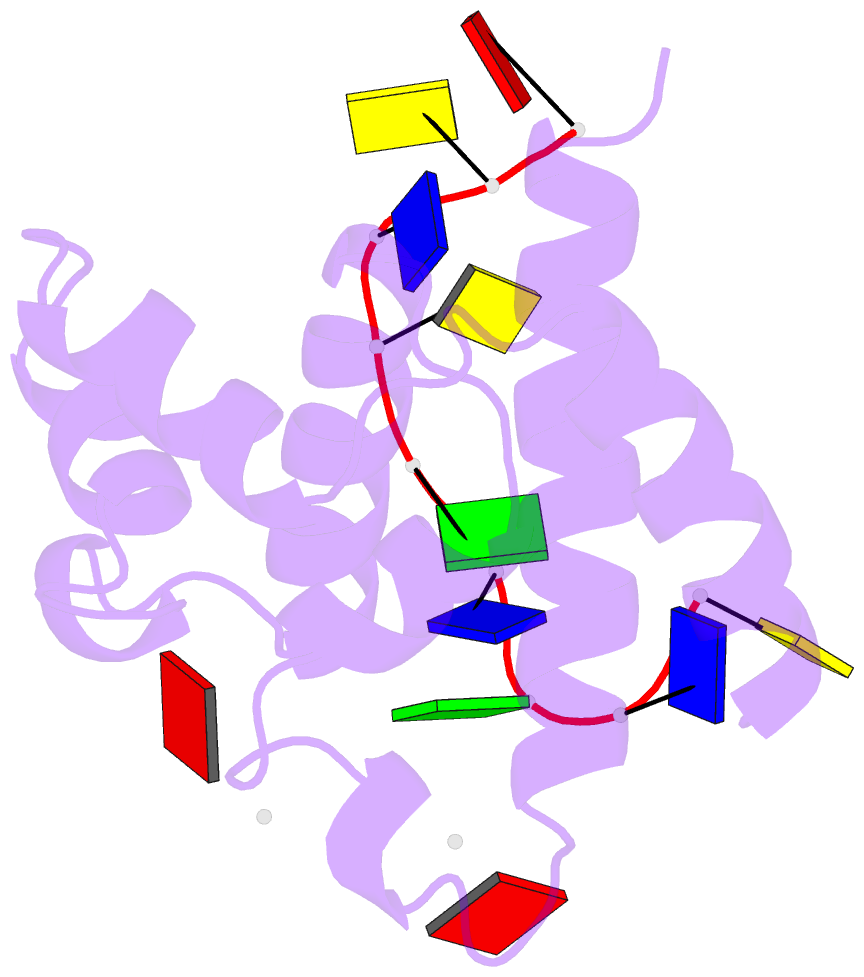
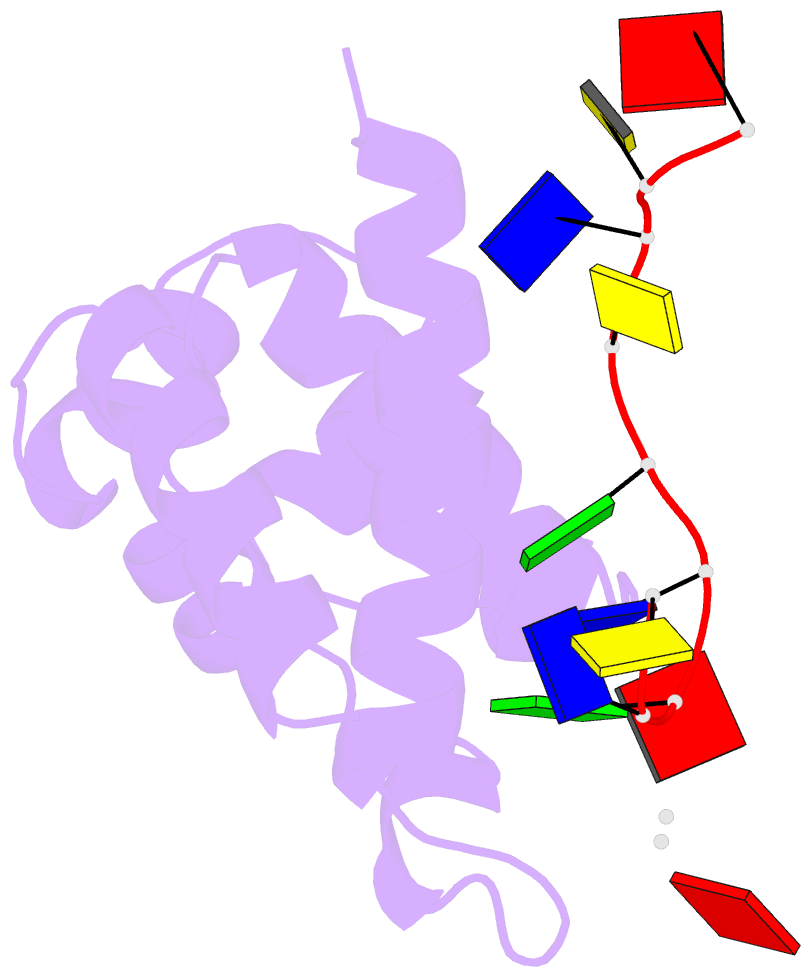
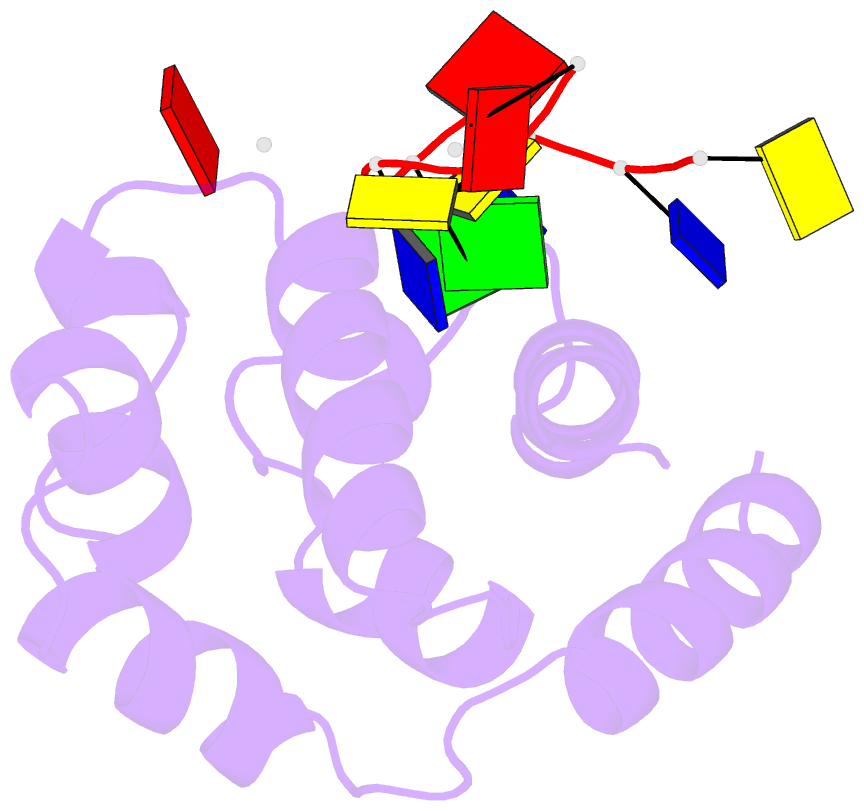
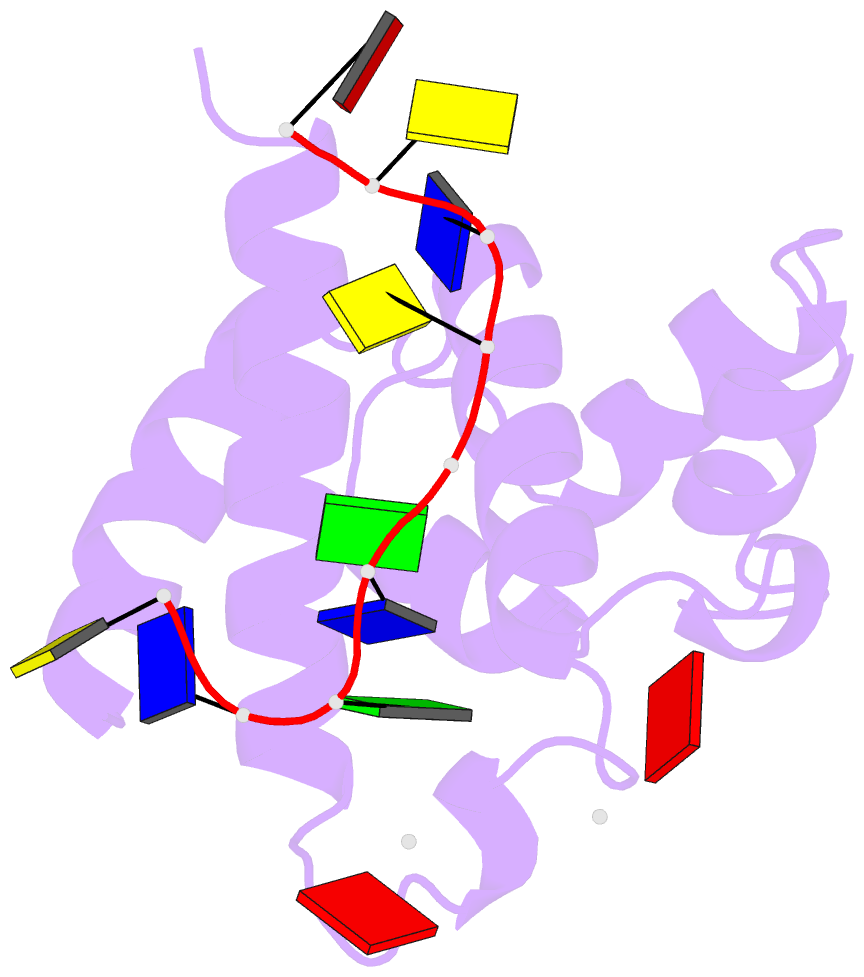
Summary information and primary citation
- PDB-id
- 6gvt; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein
- Method
- multiple methods: solution nmr, solid-state nmr
- Summary
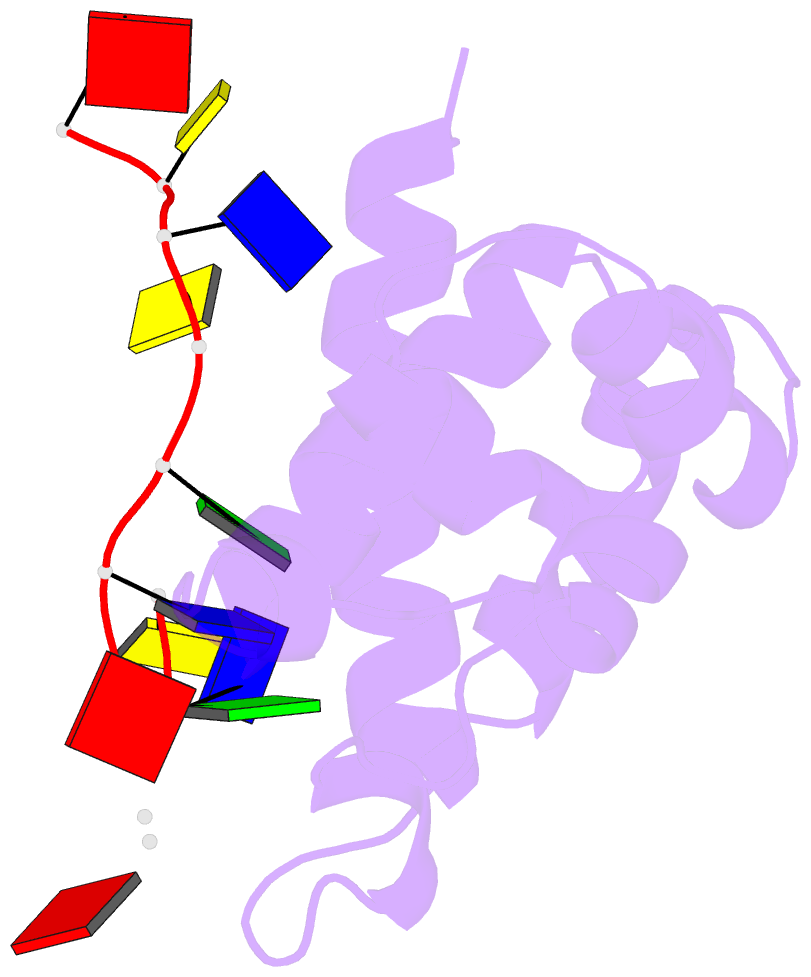
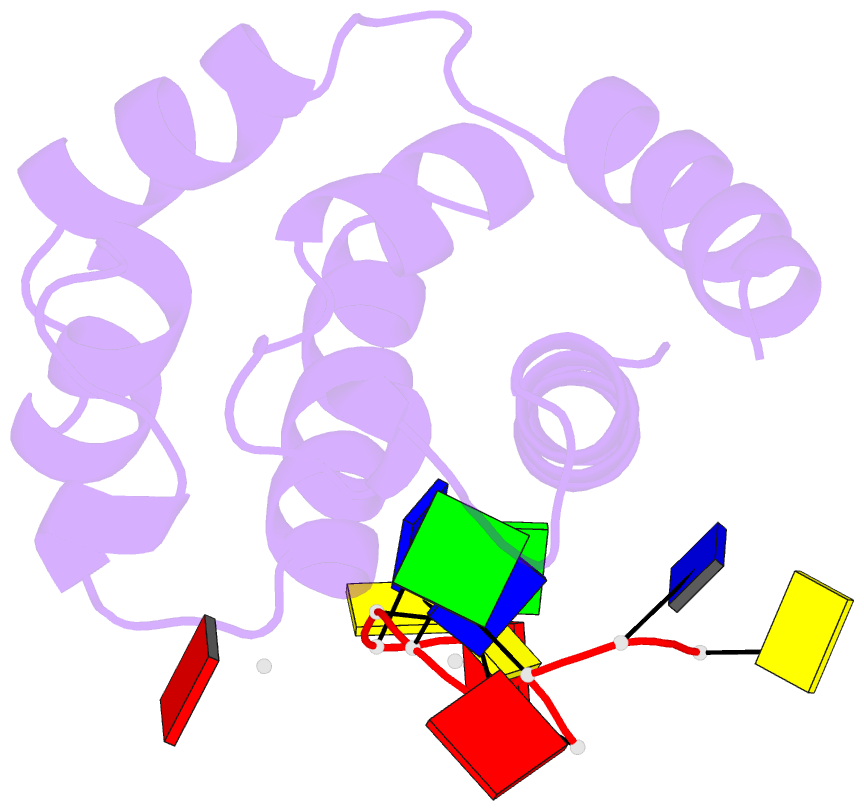
- Hybrid structure of the prn1 helix bundle domain in complex with DNA and 2 atp molecules
- Reference
- Boudet J, Devillier JC, Wiegand T, Salmon L, Meier BH, Lipps G, Allain FH (2019): "A Small Helical Bundle Prepares Primer Synthesis by Binding Two Nucleotides that Enhance Sequence-Specific Recognition of the DNA Template." Cell, 176, 154-166.e13. doi: 10.1016/j.cell.2018.11.031.
- Abstract
- Primases have a fundamental role in DNA replication. They synthesize a primer that is then extended by DNA polymerases. Archaeoeukaryotic primases require for synthesis a catalytic and an accessory domain, the exact contribution of the latter being unresolved. For the pRN1 archaeal primase, this domain is a 115-amino acid helix bundle domain (HBD). Our structural investigations of this small HBD by liquid- and solid-state nuclear magnetic resonance (NMR) revealed that only the HBD binds the DNA template. DNA binding becomes sequence-specific after a major allosteric change in the HBD, triggered by the binding of two nucleotide triphosphates. The spatial proximity of the two nucleotides and the DNA template in the quaternary structure of the HBD strongly suggests that this small domain brings together the substrates to prepare the first catalytic step of primer synthesis. This efficient mechanism is likely general for all archaeoeukaryotic primases.