Summary information and primary citation
- PDB-id
- 6hyu; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein
- Method
- X-ray (3.22 Å)
- Summary
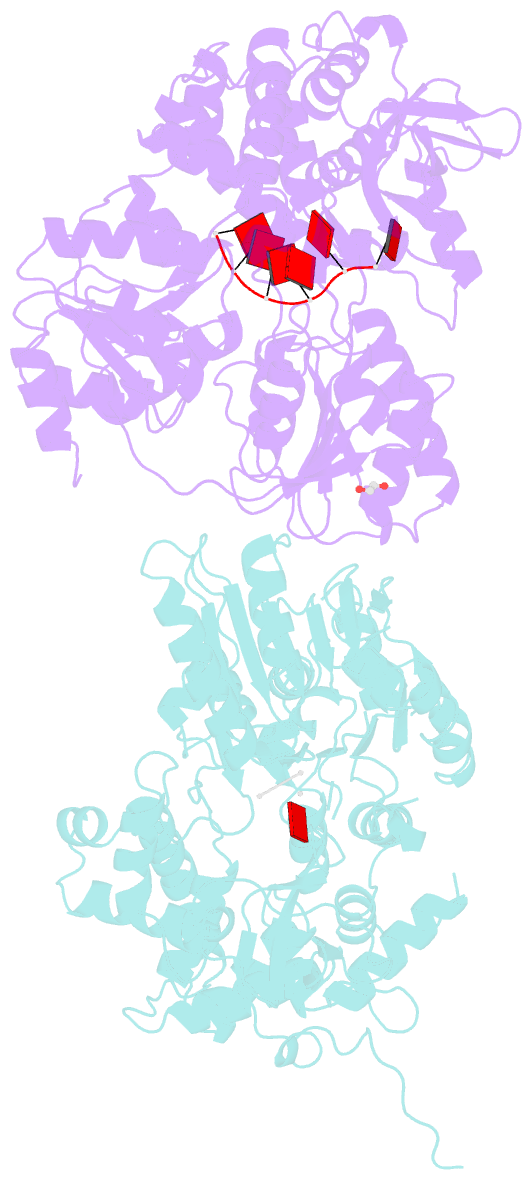
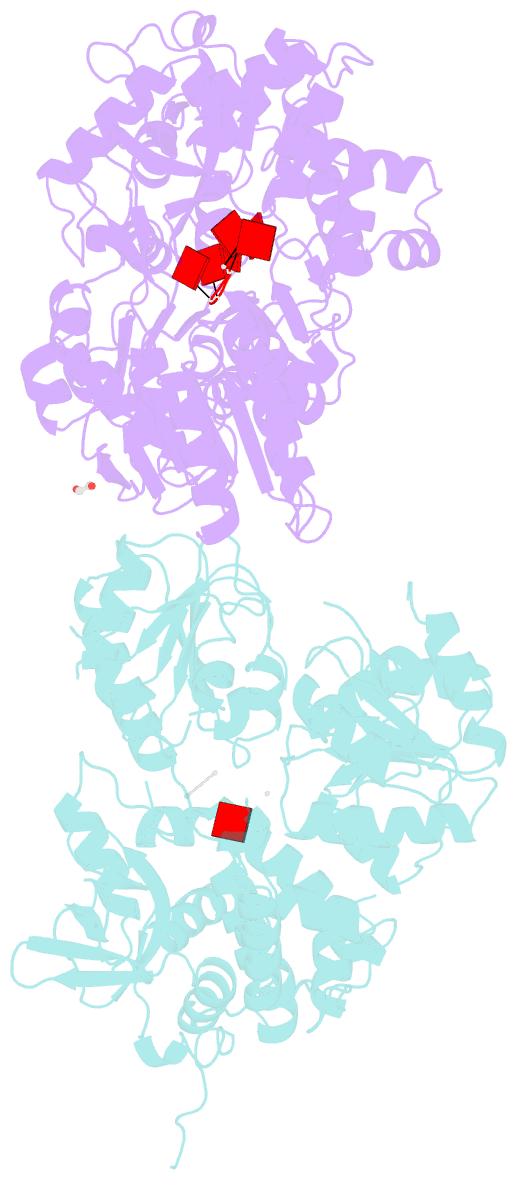
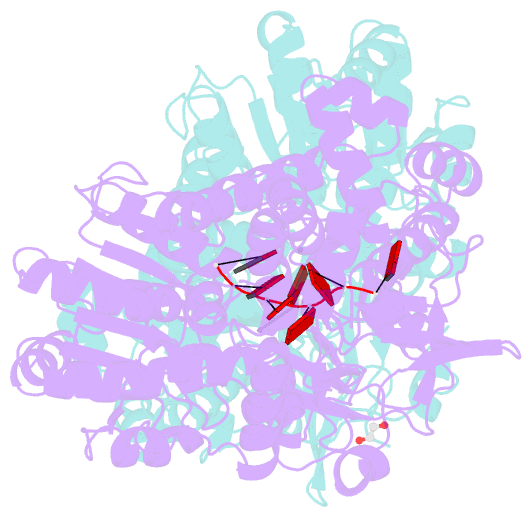
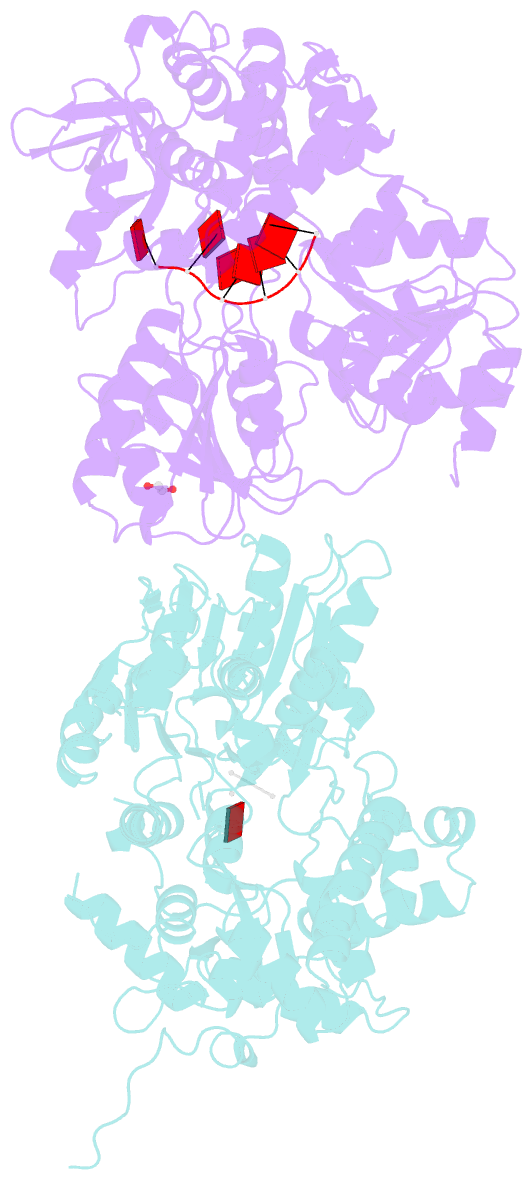
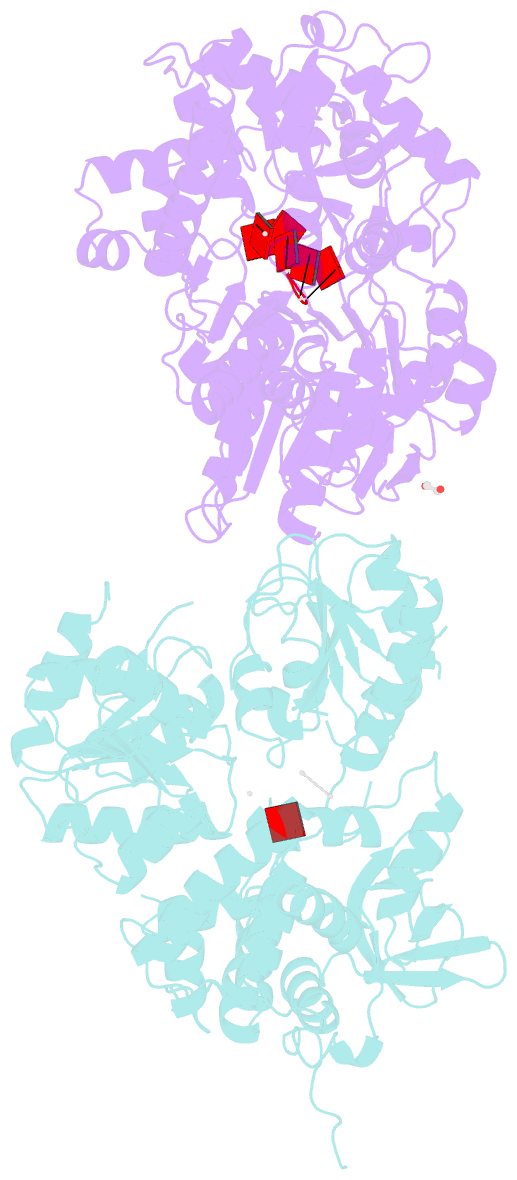
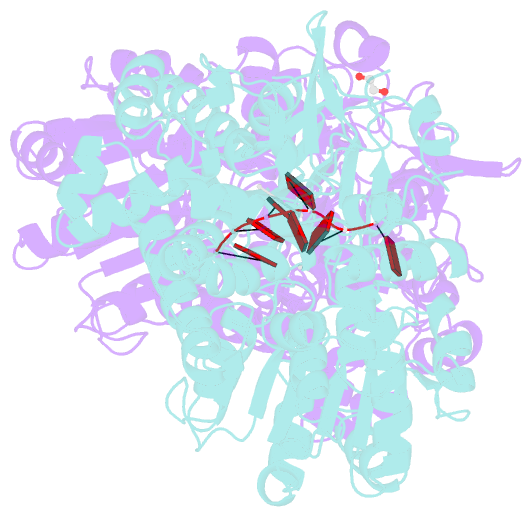
- Crystal structure of dhx8 helicase bound to single stranded poly-adenine RNA
- Reference
- Felisberto-Rodrigues C, Thomas JC, McAndrew C, Le Bihan YV, Burke R, Workman P, van Montfort RLM (2019): "Structural and functional characterisation of human RNA helicase DHX8 provides insights into the mechanism of RNA-stimulated ADP release." Biochem.J., 476, 2521-2543. doi: 10.1042/BCJ20190383.
- Abstract
- DHX8 is a crucial DEAH-box RNA helicase involved in splicing and required for the release of mature mRNA from the spliceosome. Here, we report the biochemical characterisation of full-length human DHX8 and the catalytically active helicase core DHX8Δ547, alongside crystal structures of DHX8Δ547 bound to ADP and a structure of DHX8Δ547 bound to poly(A)6 single-strand RNA. Our results reveal that DHX8 has an in vitro binding preference for adenine-rich RNA and that RNA binding triggers the release of ADP through significant conformational flexibility in the conserved DEAH-, P-loop and hook-turn motifs. We demonstrate the importance of R620 and both the hook-turn and hook-loop regions for DHX8 helicase activity and propose that the hook-turn acts as a gatekeeper to regulate the directional movement of the 3' end of RNA through the RNA-binding channel. This study provides an in-depth understanding of the activity of DHX8 and contributes insights into the RNA-unwinding mechanisms of the DEAH-box helicase family.