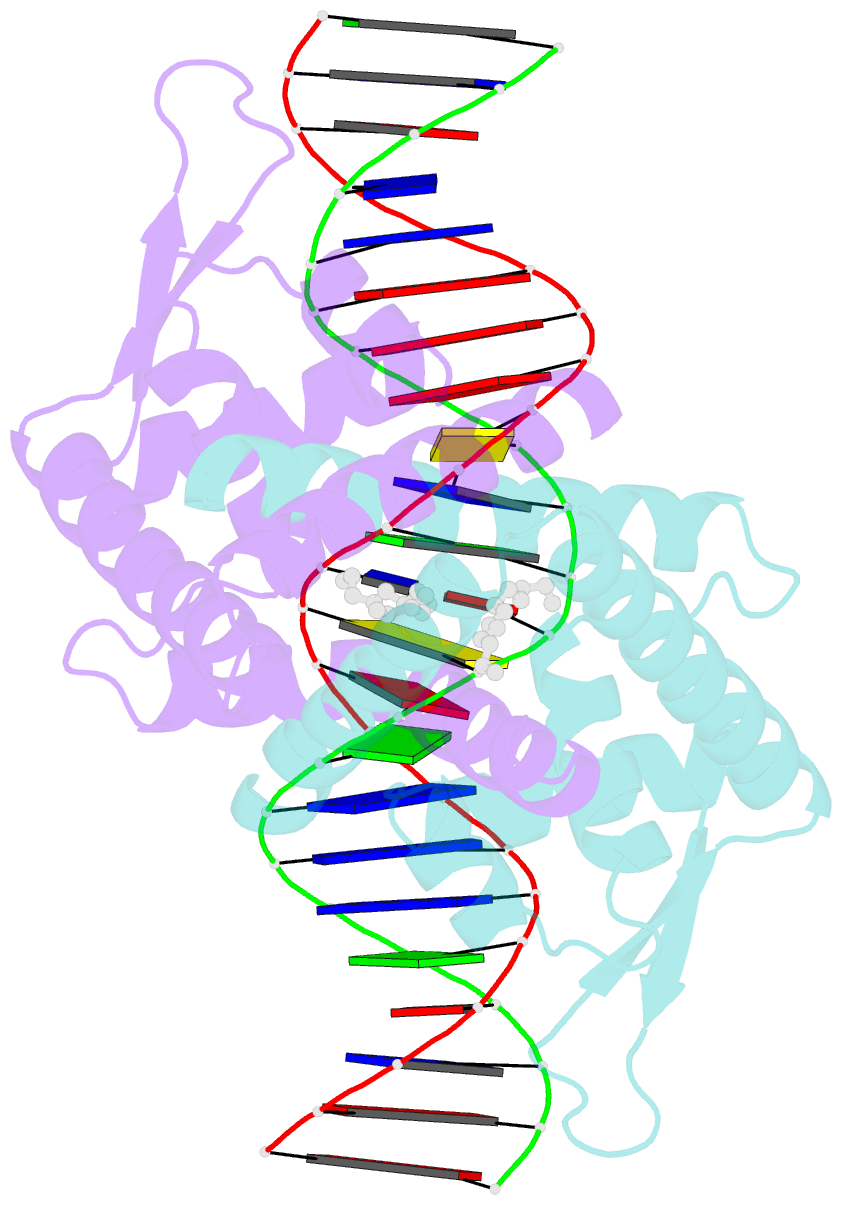
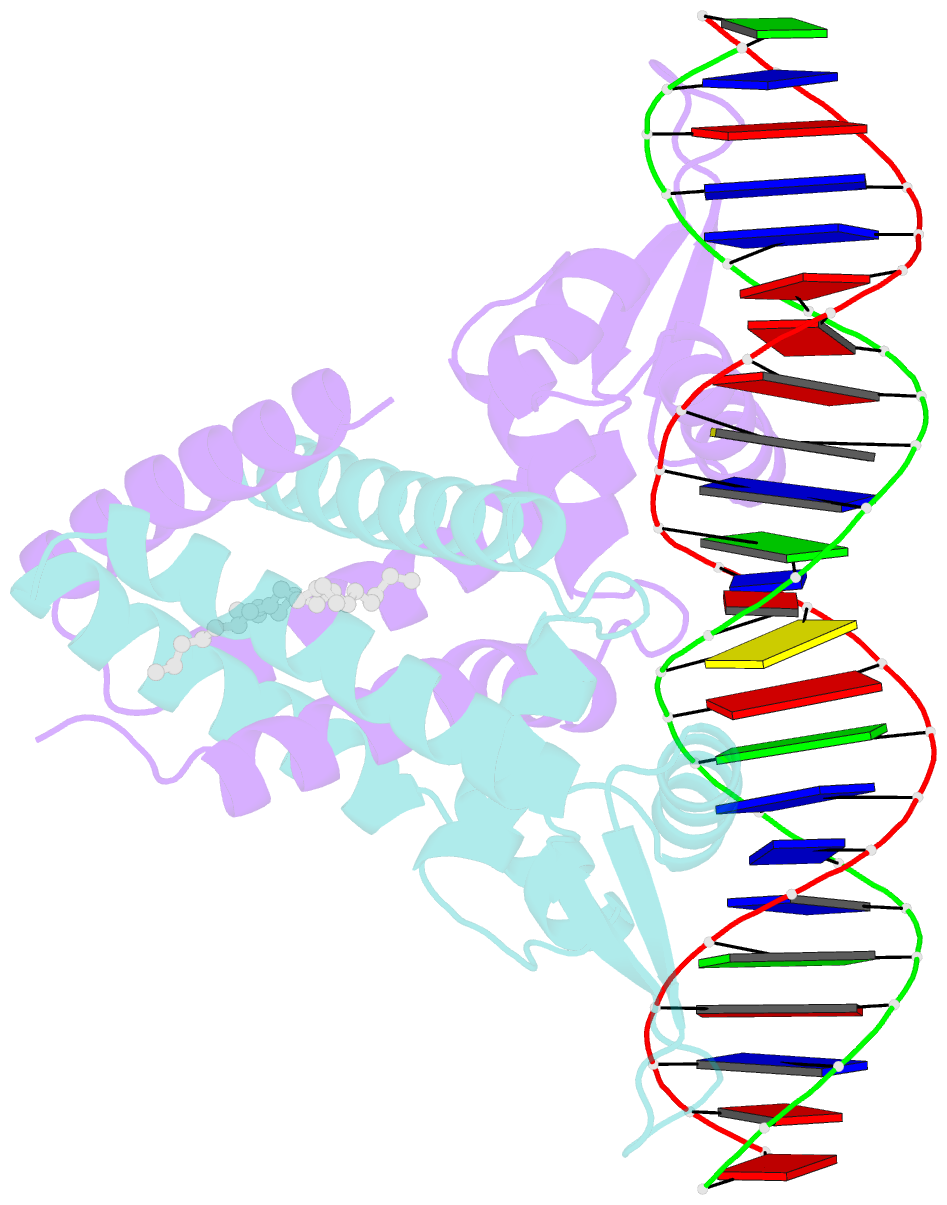
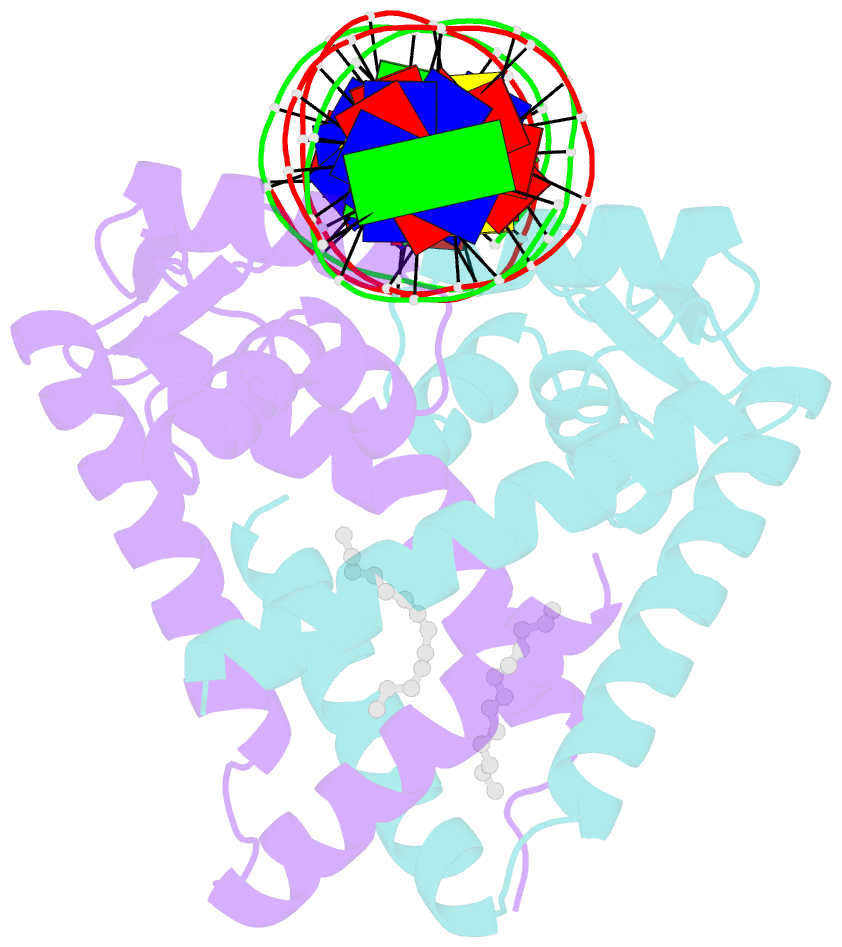
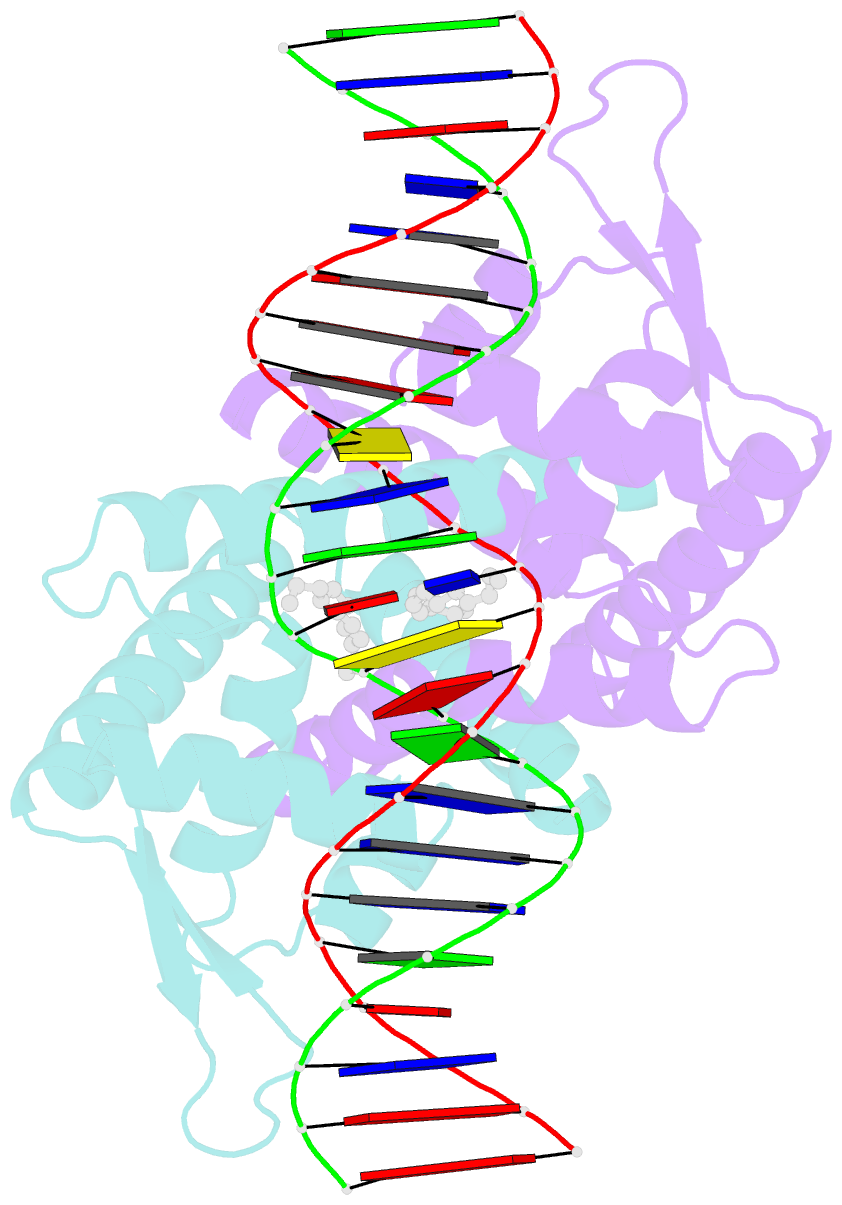
Summary information and primary citation
- PDB-id
- 6jbx; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (2.2 Å)
- Summary
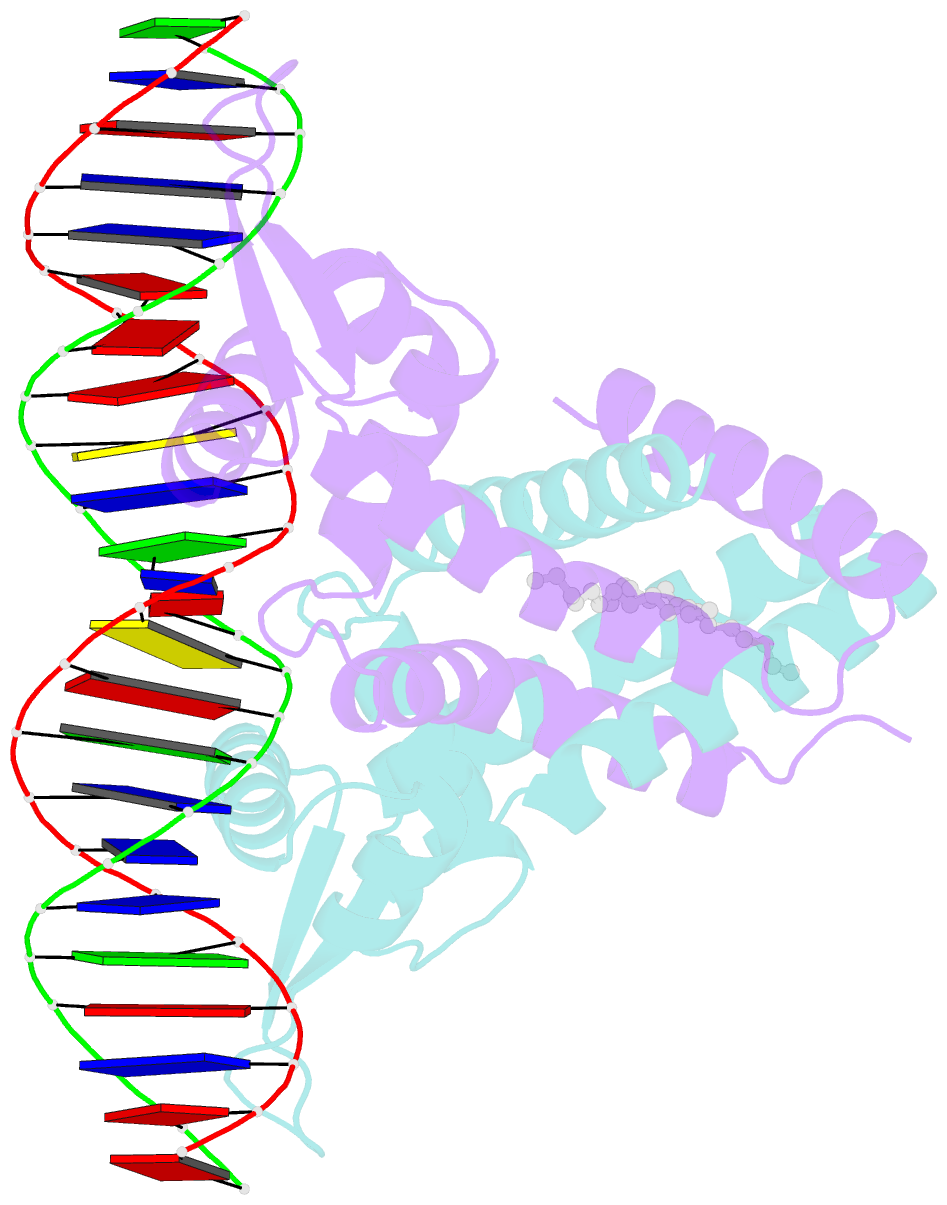
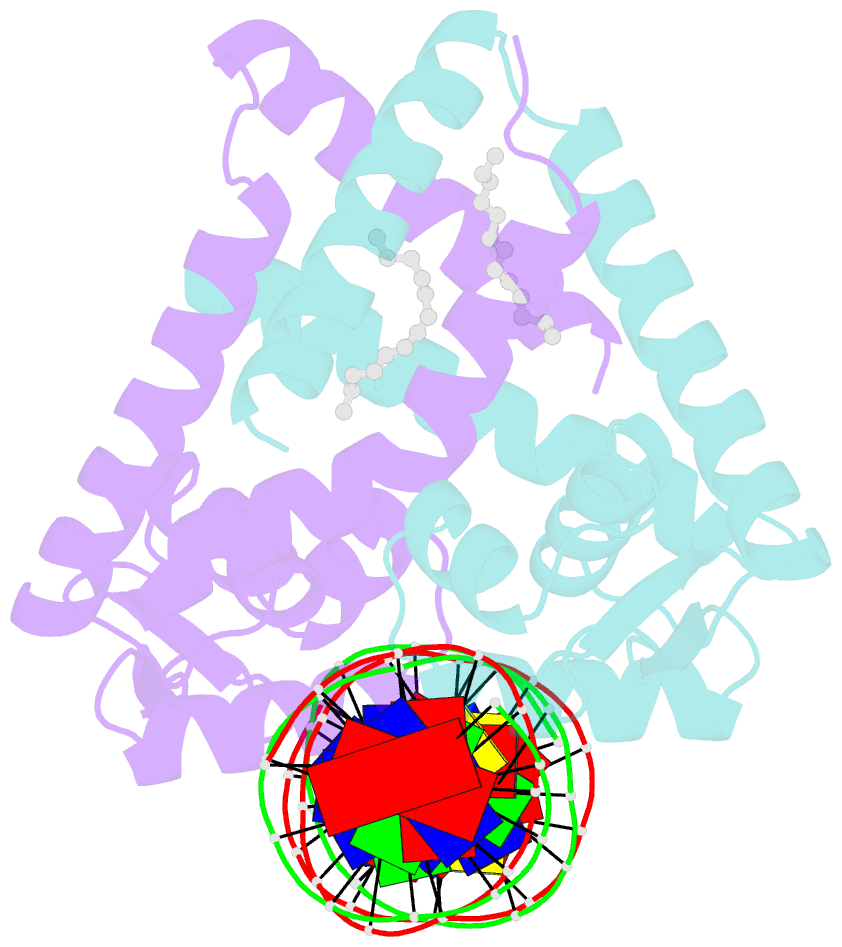
- Crystal structure of streptococcus pneumoniae fabt in complex with DNA
- Reference
- Zuo G, Chen ZP, Jiang YL, Zhu Z, Ding C, Zhang Z, Chen Y, Zhou CZ, Li Q (2019): "Structural insights into repression of the Pneumococcal fatty acid synthesis pathway by repressor FabT and co-repressor acyl-ACP." Febs Lett., 593, 2730-2741. doi: 10.1002/1873-3468.13534.
- Abstract
- The Streptococcus pneumoniae fatty acid synthesis (FAS) pathway is globally controlled at the transcriptional level by the repressor FabT and its co-repressor acyl carrier protein (acyl-ACP), the intermediate of phospholipid synthesis. Here, we report the crystal structure of FabT complexed with a 23-bp dsDNA, which indicates that FabT is a weak repressor with low DNA-binding affinity in the absence of acyl-ACP. Modification of ACP with a long-chain fatty acid is necessary for the formation of a stable complex with FabT, mimicked in vitro by cross-linking, which significantly elevates the DNA-binding affinity of FabT. Altogether, we propose a putative working model of gene repression under the double control of FabT and acyl-ACP, elucidating a distinct repression network for Pneumococcus to precisely coordinate FAS.