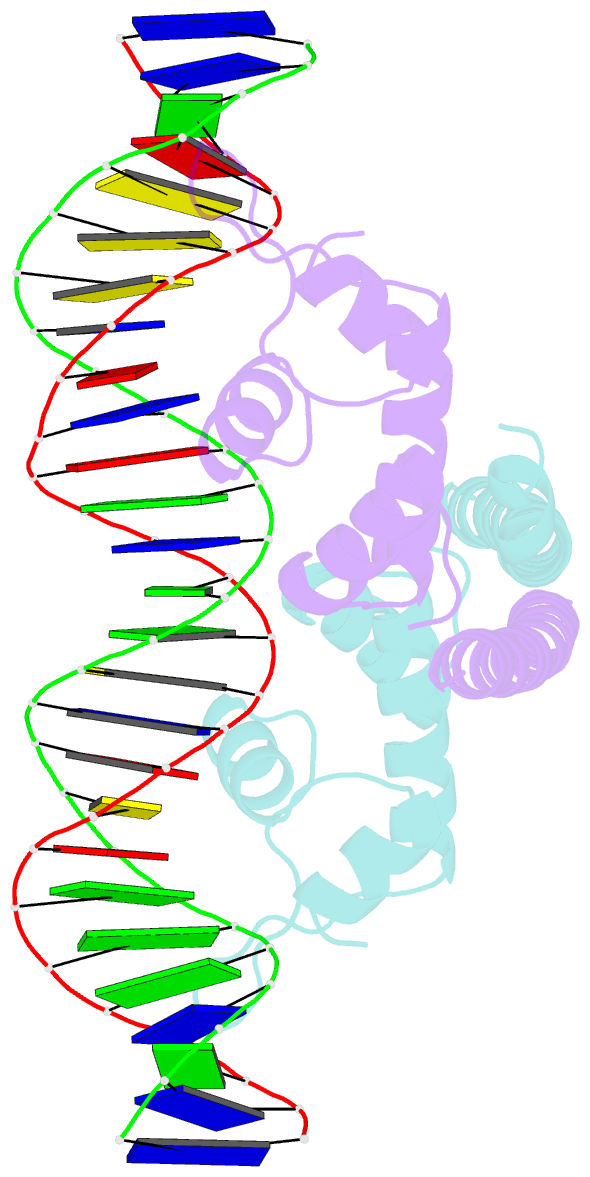
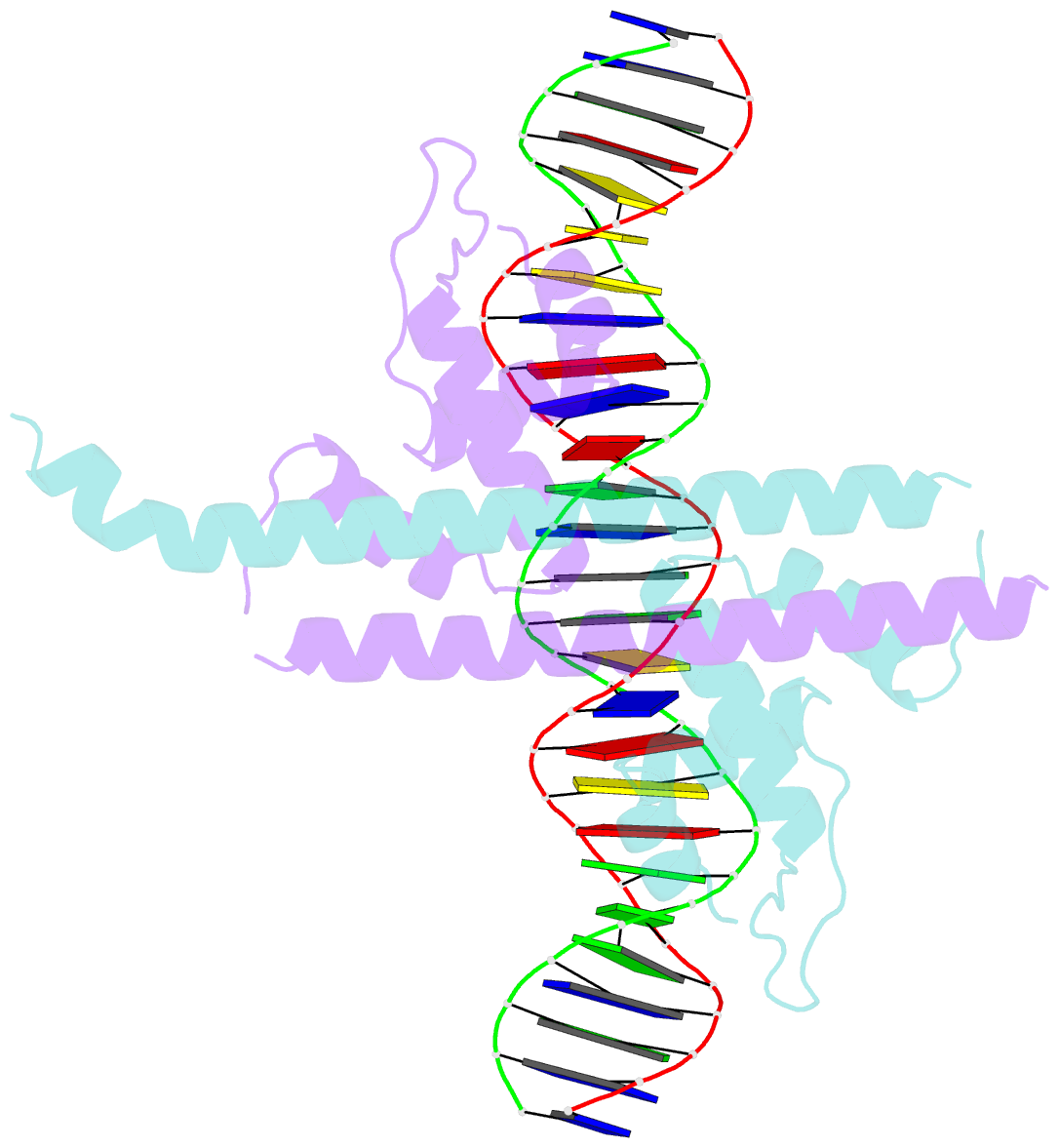
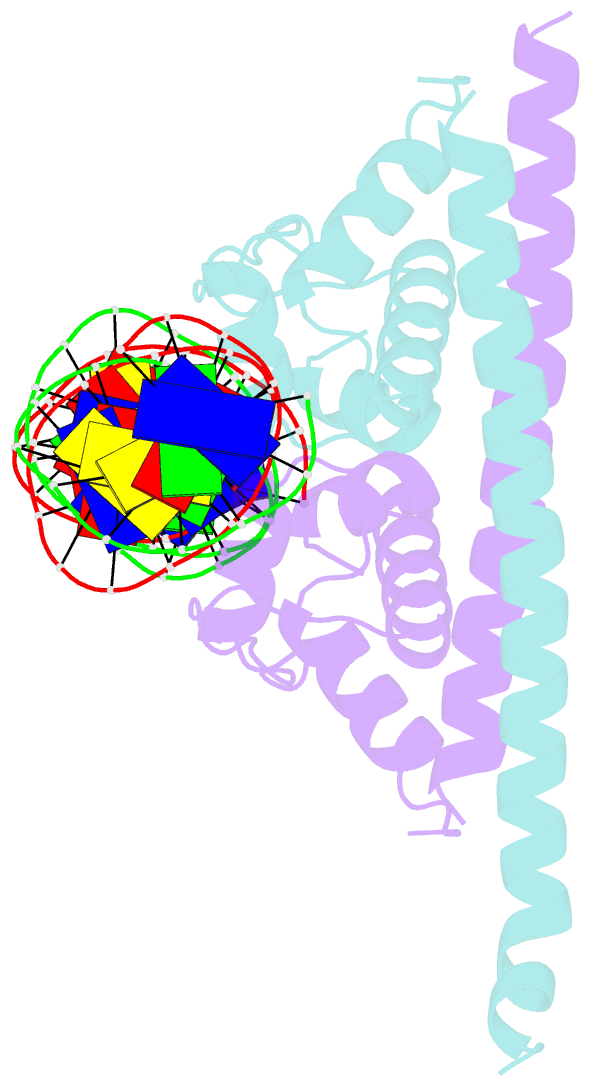
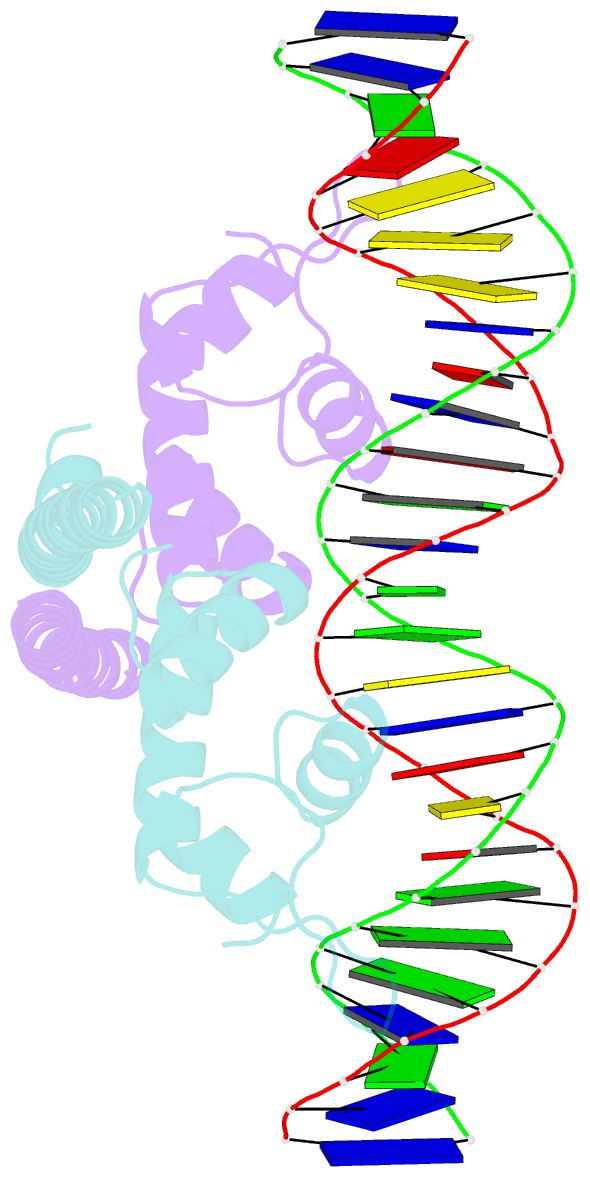
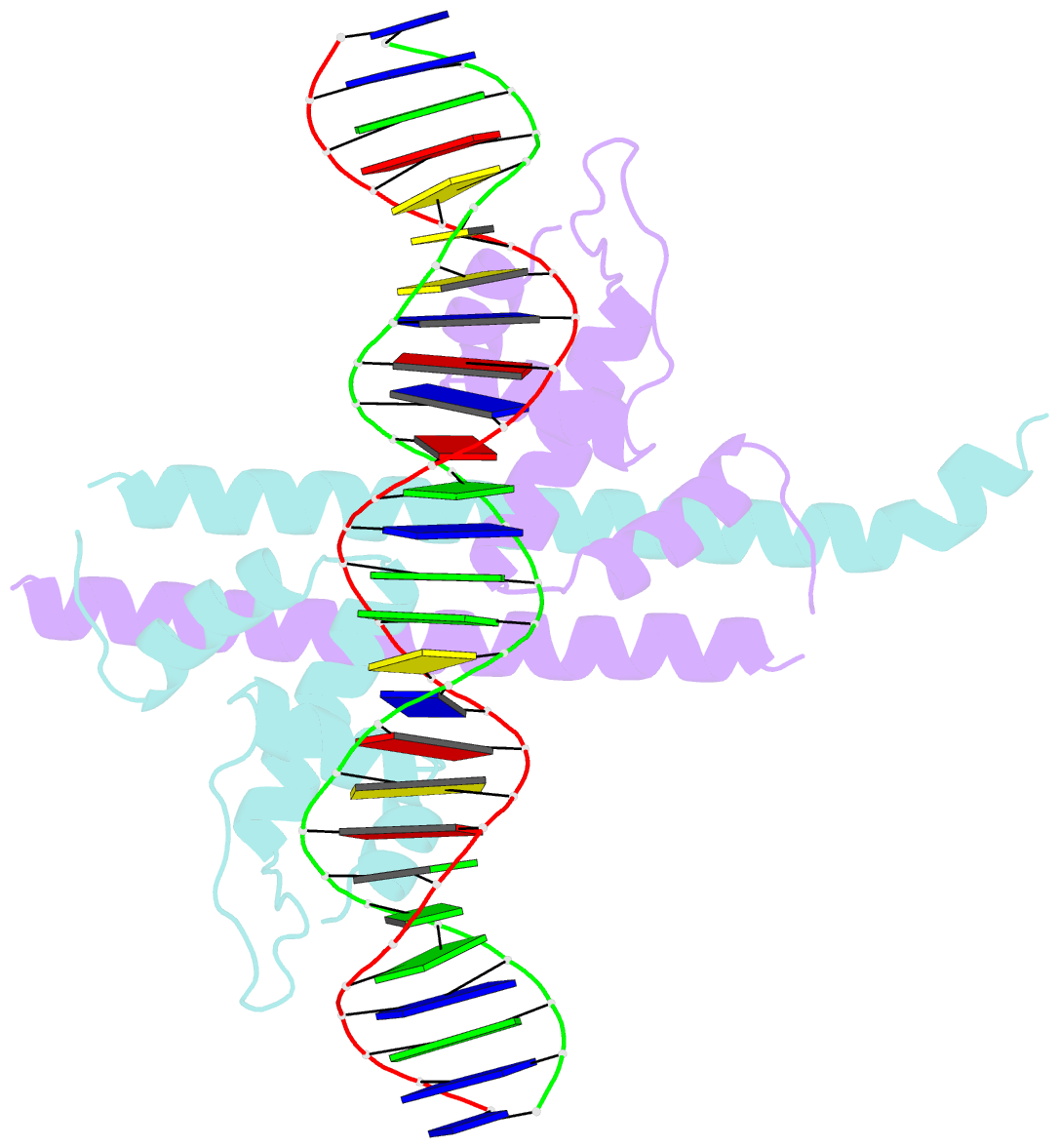
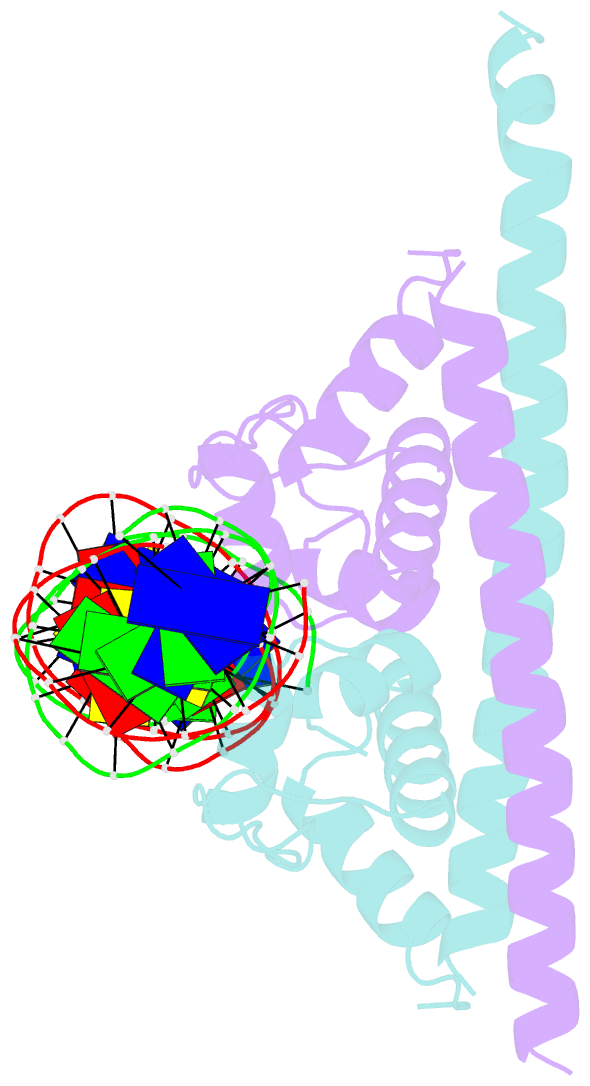
Summary information and primary citation
- PDB-id
- 6jgw; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (2.8 Å)
- Summary
- Crystal structure of the transcriptional regulator cadr from p. putida in complex with DNA
- Reference
- Liu X, Hu Q, Yang J, Huang S, Wei T, Chen W, He Y, Wang D, Liu Z, Wang K, Gan J, Chen H (2019): "Selective cadmium regulation mediated by a cooperative binding mechanism in CadR." Proc.Natl.Acad.Sci.USA, 116, 20398-20403. doi: 10.1073/pnas.1908610116.
- Abstract
- Detoxification of the highly toxic cadmium element is essential for the survival of living organisms. Pseudomonas putida CadR, a MerR family transcriptional regulator, has been reported to exhibit an ultraspecific response to the cadmium ion. Our crystallographic and spectroscopic studies reveal that the extra cadmium selectivity of CadR is mediated by the unexpected cooperation of thiolate-rich site I and histidine-rich site II. Cadmium binding in site I mediates the reorientation of protein domains and facilitates the assembly of site II. Subsequently, site II bridge-links 2 DNA binding domains through ligands His140/His145 in the C-terminal histidine-rich tail. With dynamic transit between 2 conformational states, this bridge could stabilize the regulator into an optimal conformation that is critical for enhancing the transcriptional activity of the cadmium detoxification system. Our results provide dynamic insight into how nature utilizes the unique cooperative binding mechanism in multisite proteins to recognize cadmium ions specifically.