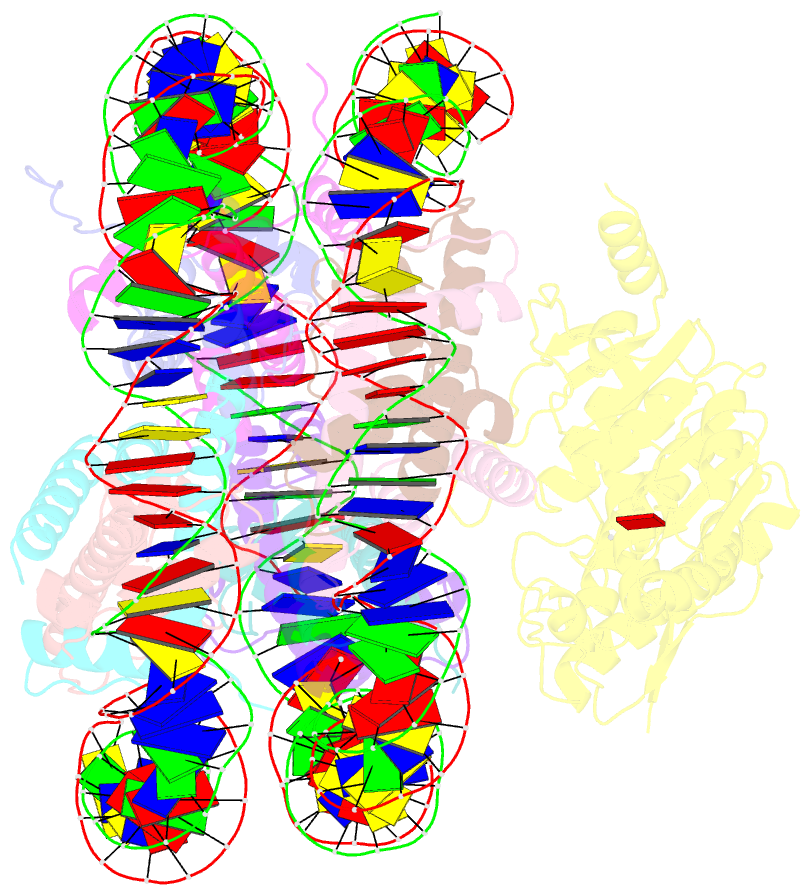
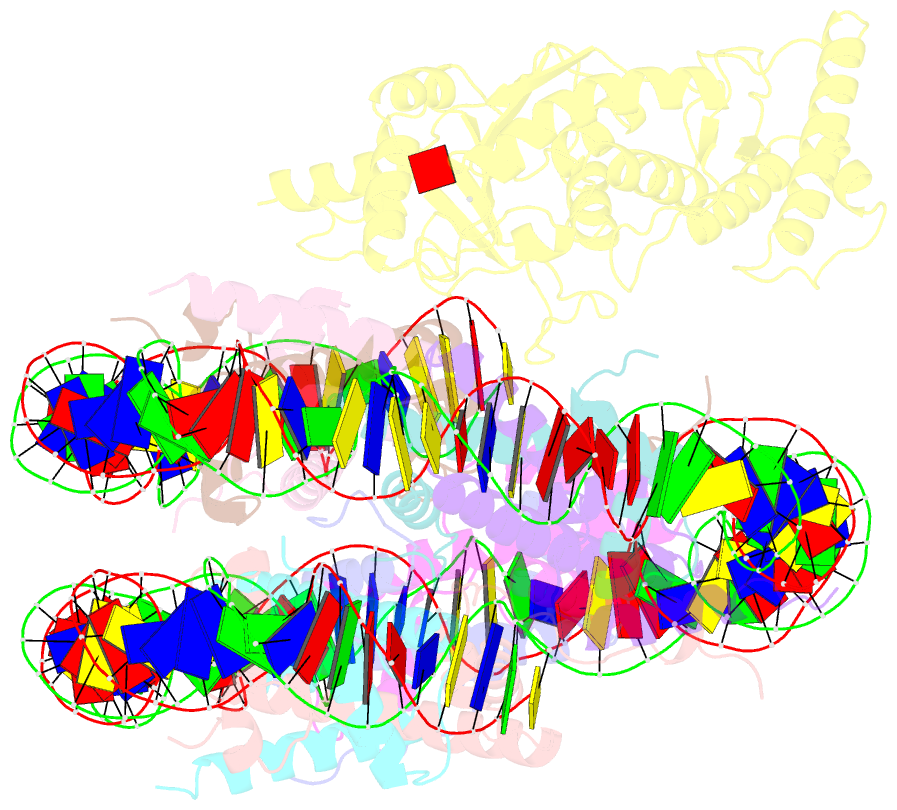
Summary information and primary citation
- PDB-id
- 6jm9; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- gene regulation
- Method
- cryo-EM (7.3 Å)
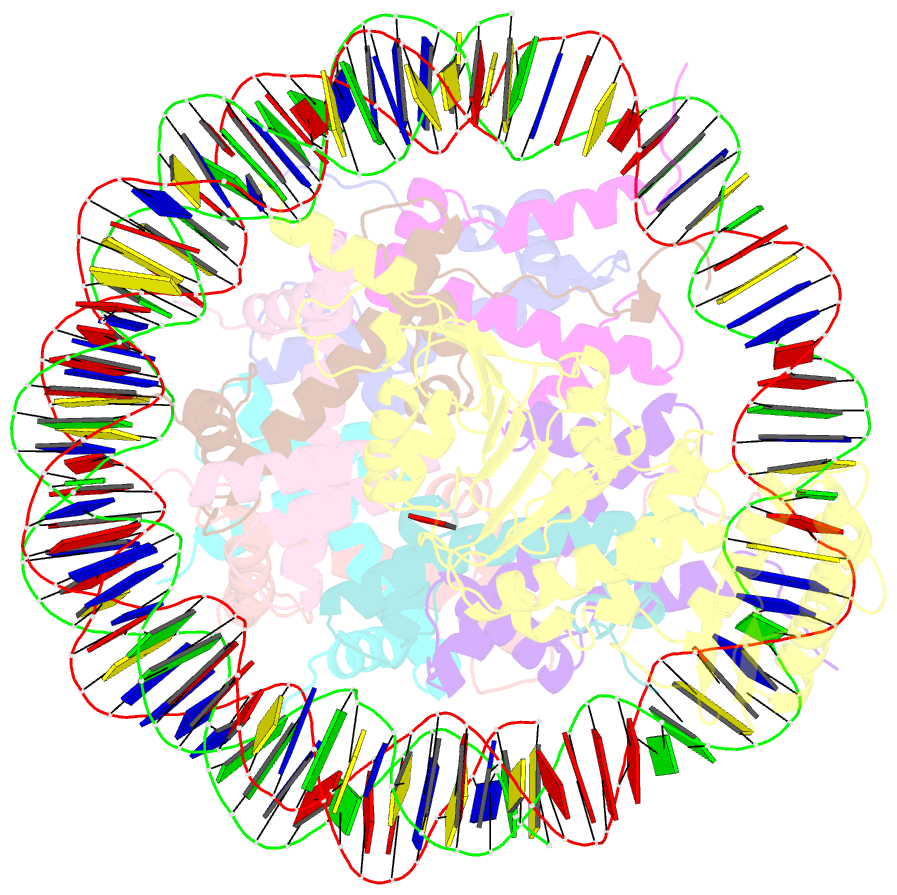
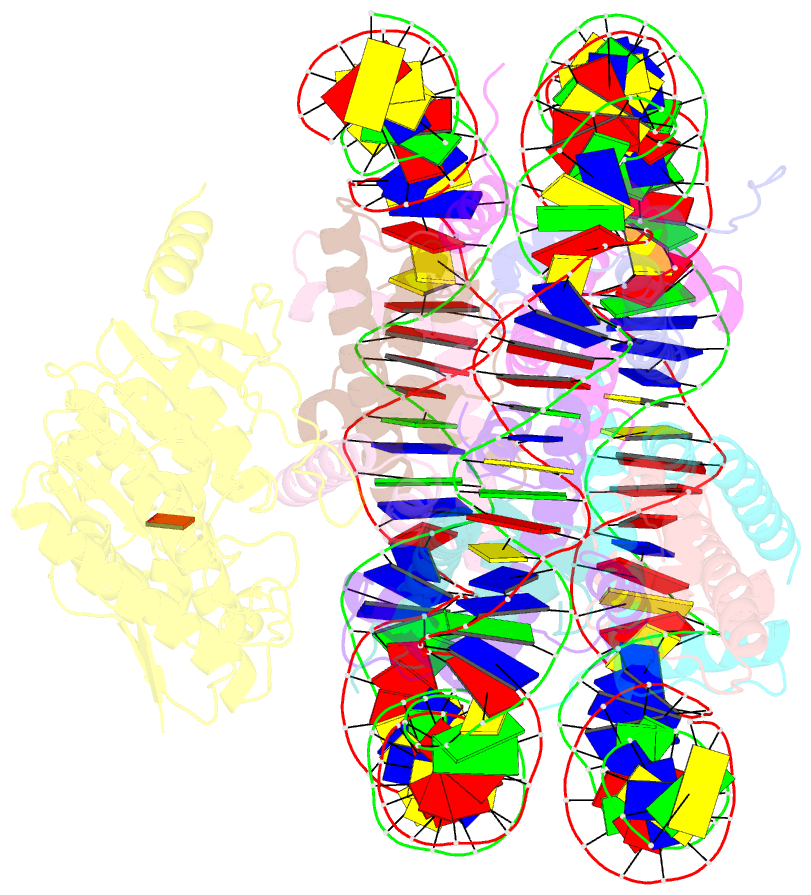
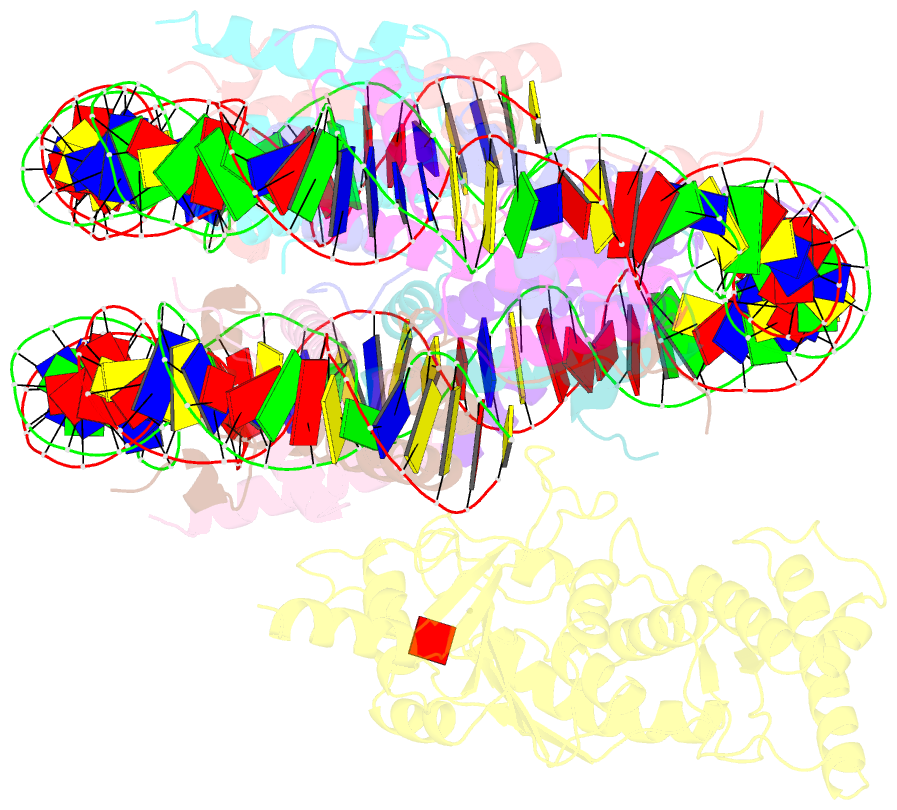
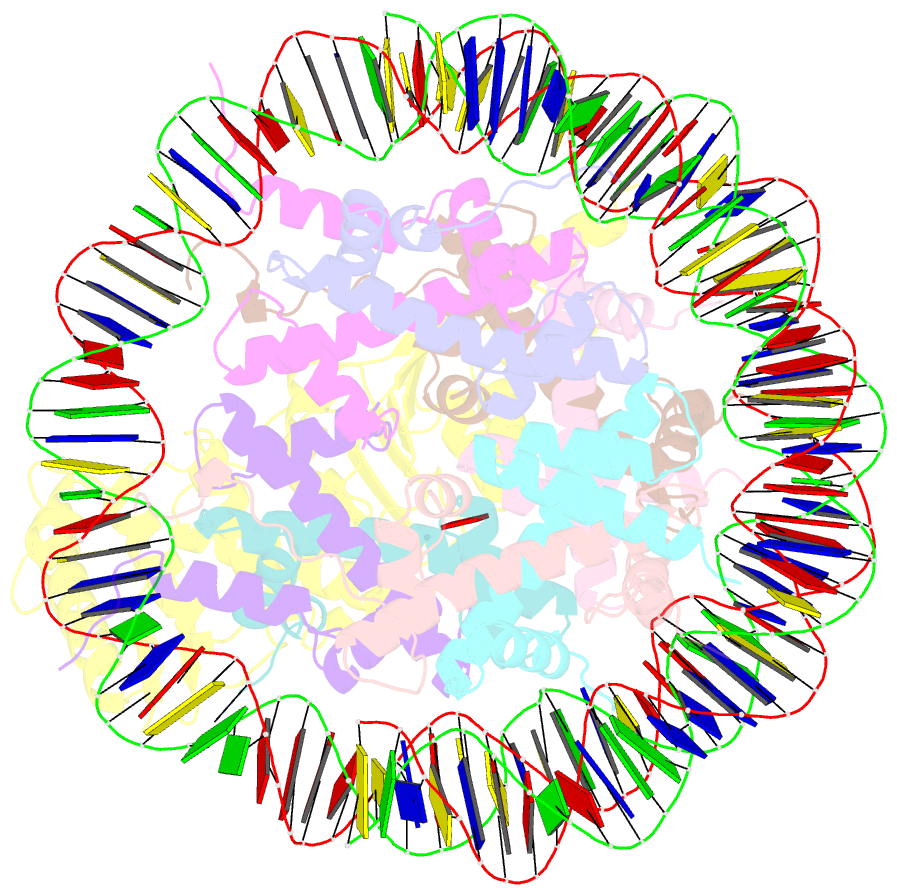
- Summary
- cryo-EM structure of dot1l bound to unmodified nucleosome
- Reference
- Jang S, Kang C, Yang HS, Jung T, Hebert H, Chung KY, Kim SJ, Hohng S, Song JJ (2019): "Structural basis of recognition and destabilization of the histone H2B ubiquitinated nucleosome by the DOT1L histone H3 Lys79 methyltransferase." Genes Dev., 33, 620-625. doi: 10.1101/gad.323790.118.
- Abstract
- DOT1L is a histone H3 Lys79 methyltransferase whose activity is stimulated by histone H2B Lys120 ubiquitination, suggesting cross-talk between histone H3 methylation and H2B ubiquitination. Here, we present cryo-EM structures of DOT1L complexes with unmodified or H2B ubiquitinated nucleosomes, showing that DOT1L recognizes H2B ubiquitin and the H2A/H2B acidic patch through a C-terminal hydrophobic helix and an arginine anchor in DOT1L, respectively. Furthermore, the structures combined with single-molecule FRET experiments show that H2B ubiquitination enhances a noncatalytic function of the DOT1L-destabilizing nucleosome. These results establish the molecular basis of the cross-talk between H2B ubiquitination and H3 Lys79 methylation as well as nucleosome destabilization by DOT1L.