Summary information and primary citation
- PDB-id
- 6jvx; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein-RNA
- Method
- X-ray (2.301 Å)
- Summary
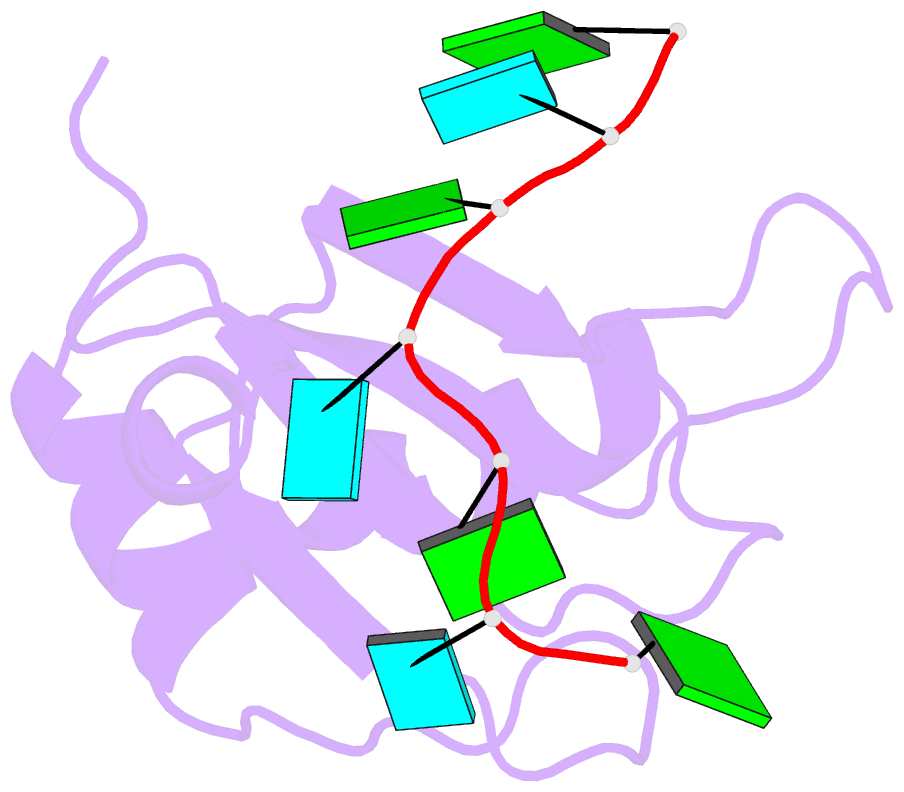
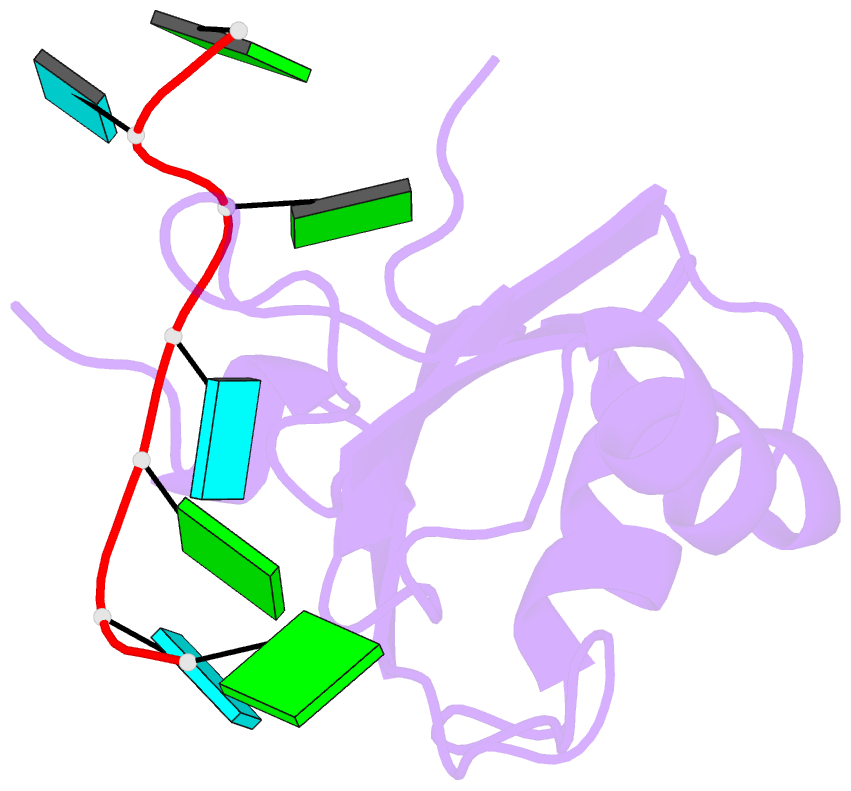
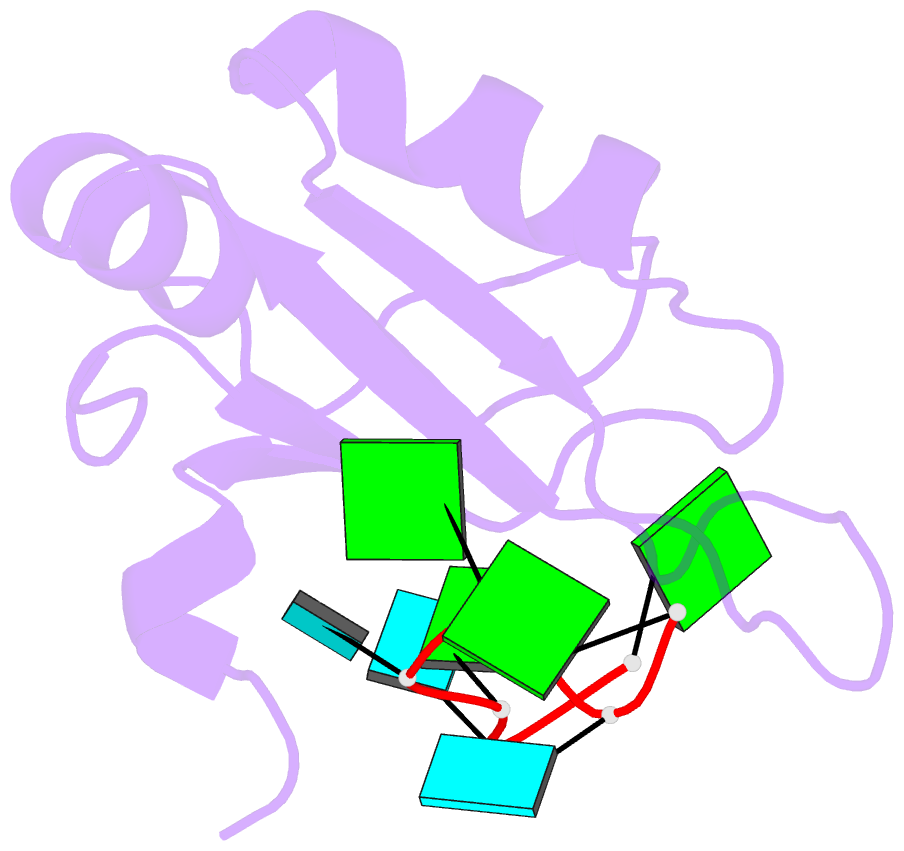
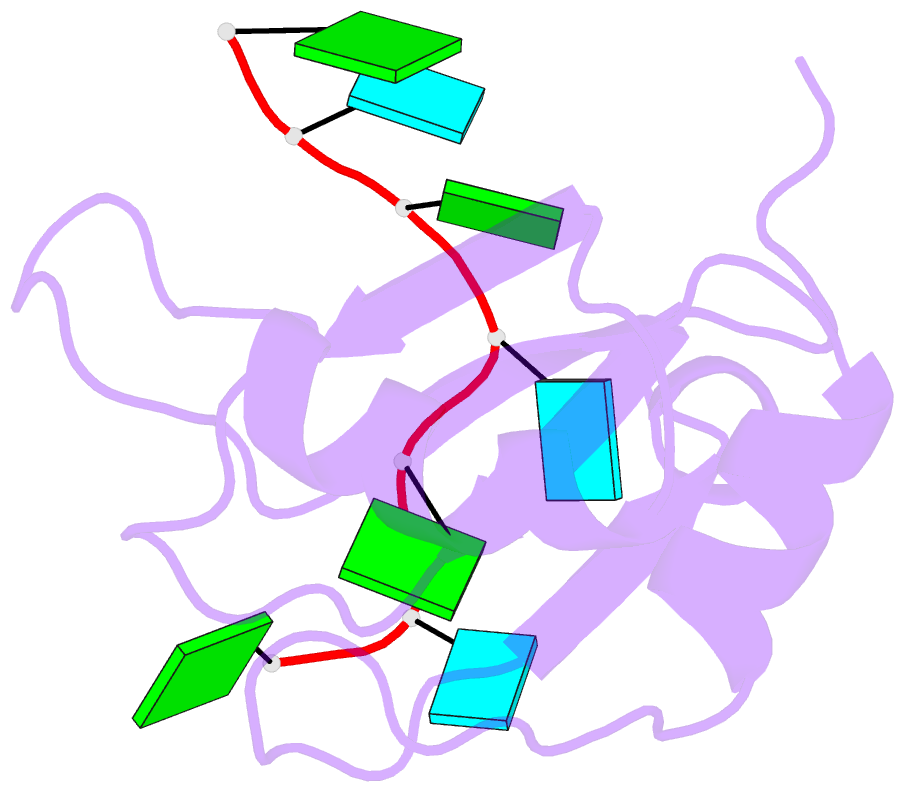
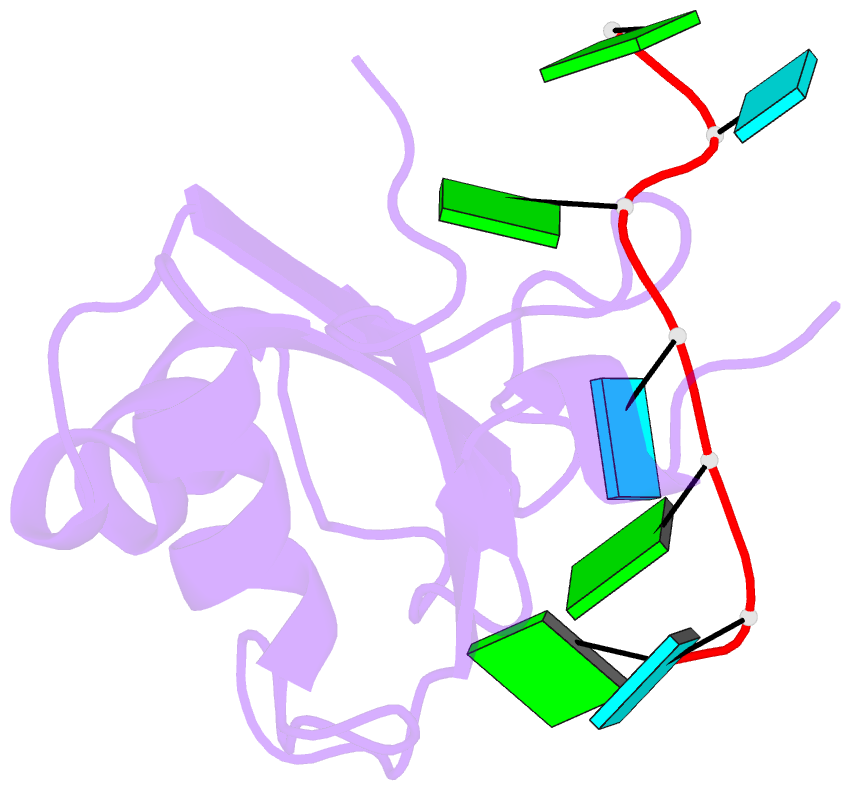
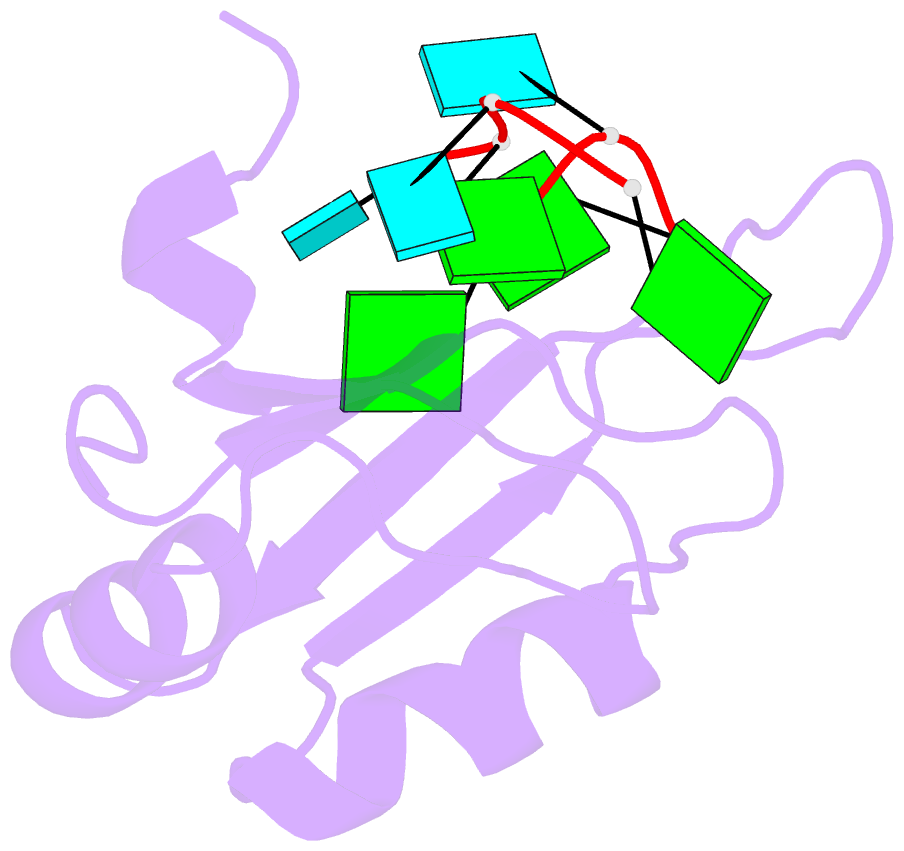
- Crystal structure of rbm38 in complex with RNA
- Reference
- Qian K, Li M, Wang J, Zhang M, Wang M (2020): "Structural basis for mRNA recognition by human RBM38." Biochem.J., 477, 161-172. doi: 10.1042/BCJ20190652.
- Abstract
- RNA-binding protein RBM38 was reported to bind the mRNA of several p53-related genes through its RRM domain and to up-regulate or down-regulate protein translation by increasing mRNA stability or recruitment of other effector proteins. The recognition mechanism, however, for RNA-binding of RBM38 remains unclear. Here, we report the crystal structure of the RRM domain of human RBM38 in complex with a single-stranded RNA. Our structural and biological results revealed that RBM38 recognizes G(U/C/A)GUG sequence single-stranded RNA in a sequence-specific and structure-specific manner. Two phenylalanine stacked with bases of RNA were crucial for RNA binding, and a series of hydrogen bonds between the base atoms of RNA and main-chain or side-chain atoms of RBM38 determine the sequence-specific recognition. Our results revealed the RNA-recognition mechanism of human RBM38 and provided structural information for understanding the RNA-binding property of RBM38.