Summary information and primary citation
- PDB-id
- 6k32; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- viral protein-RNA
- Method
- cryo-EM (3.2 Å)
- Summary
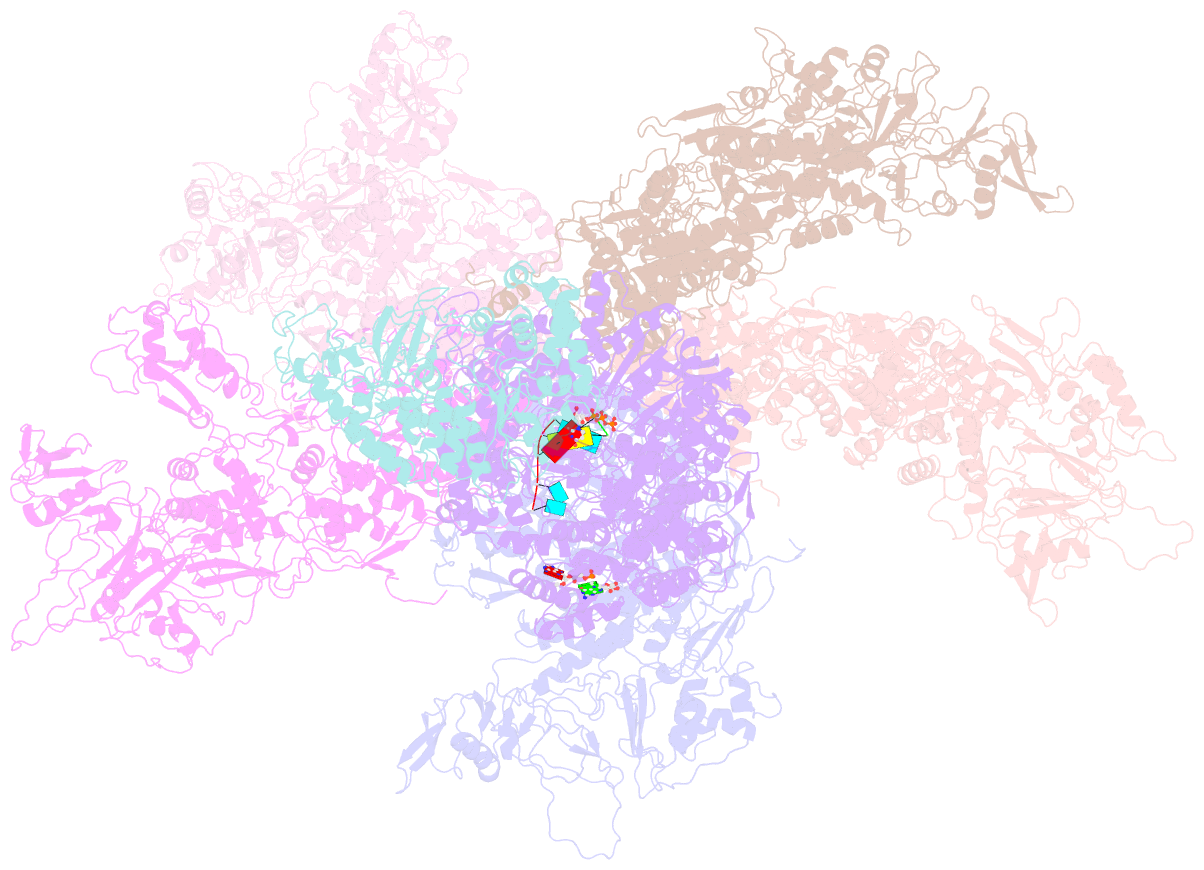
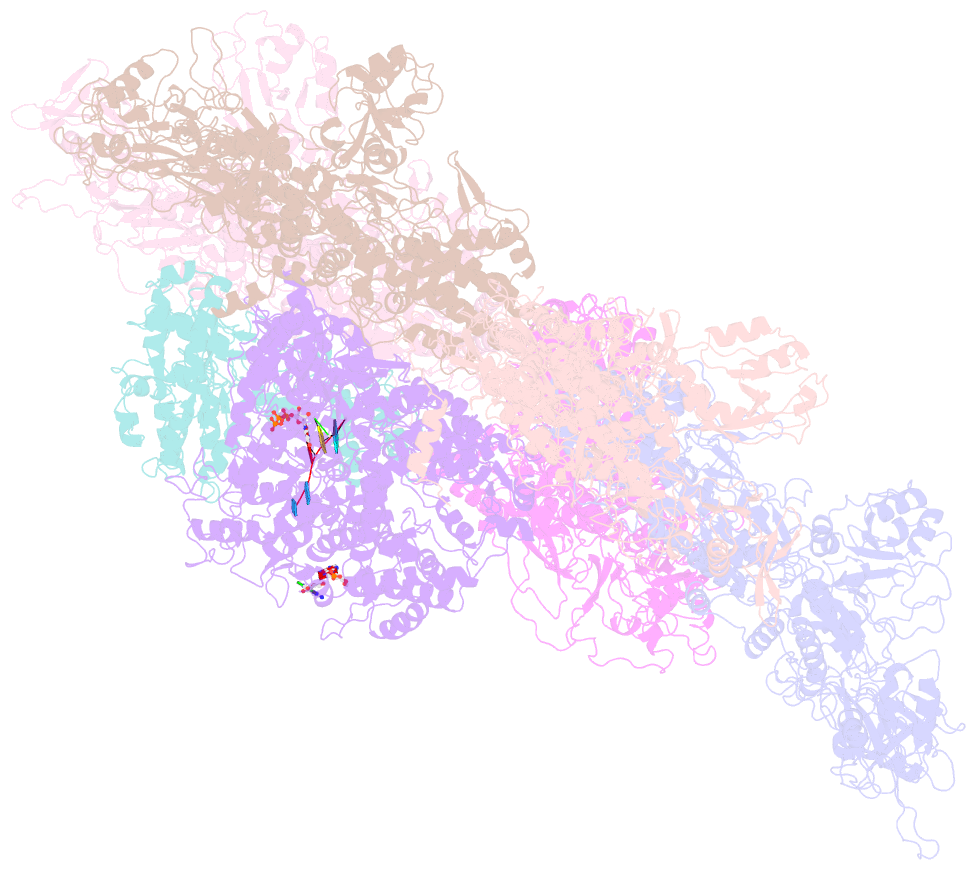
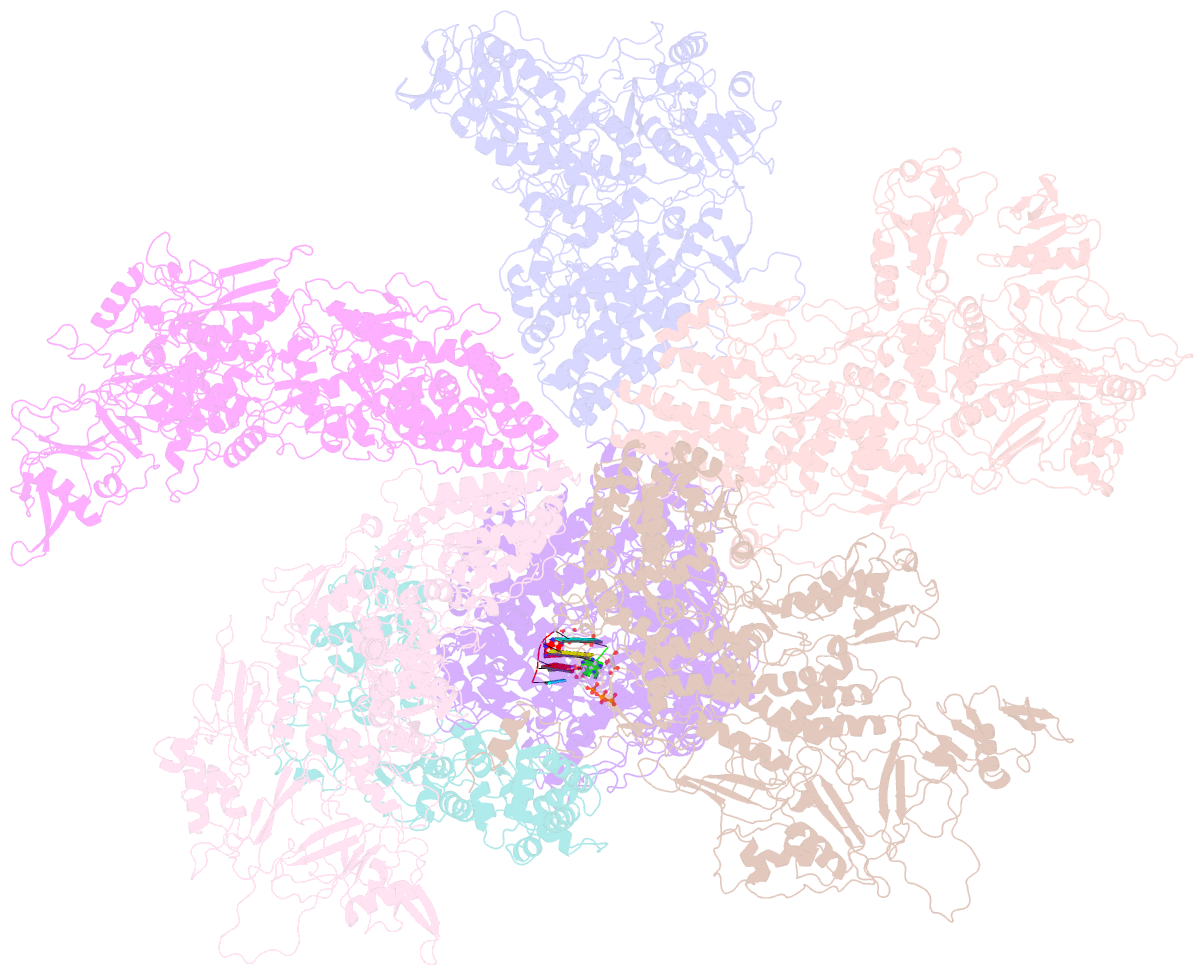
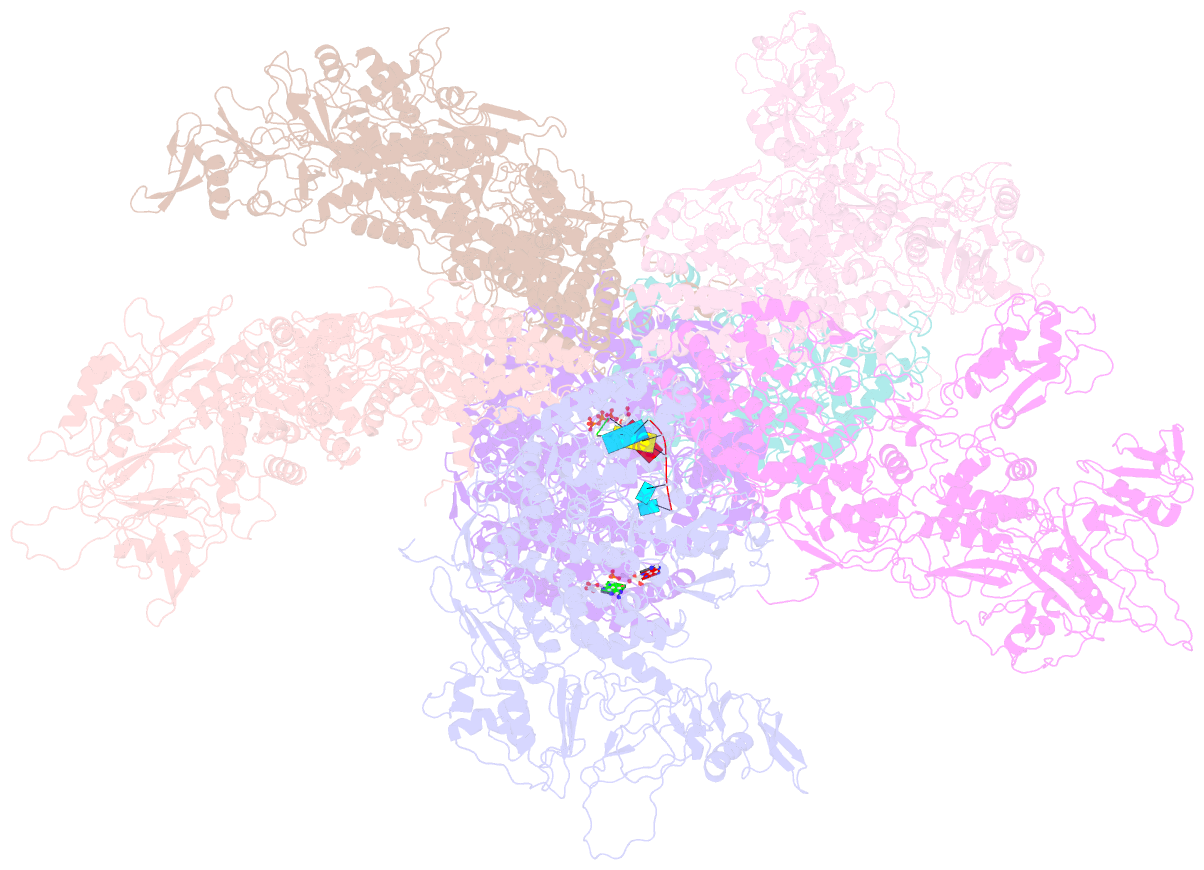
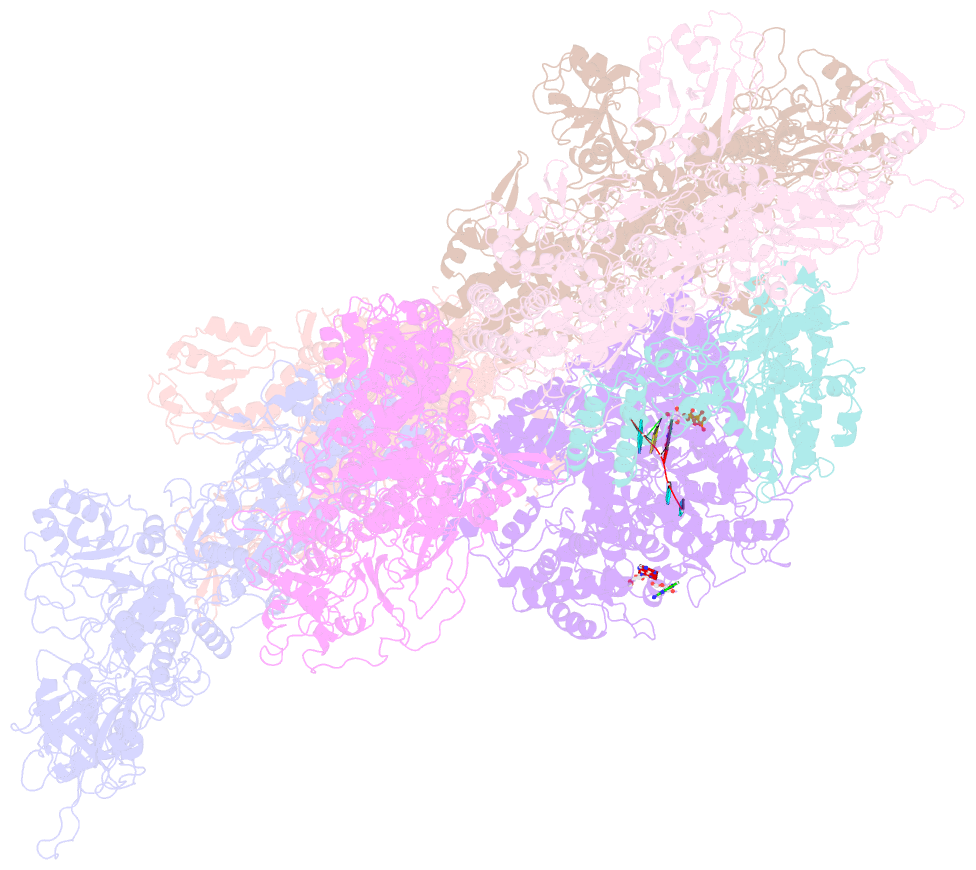
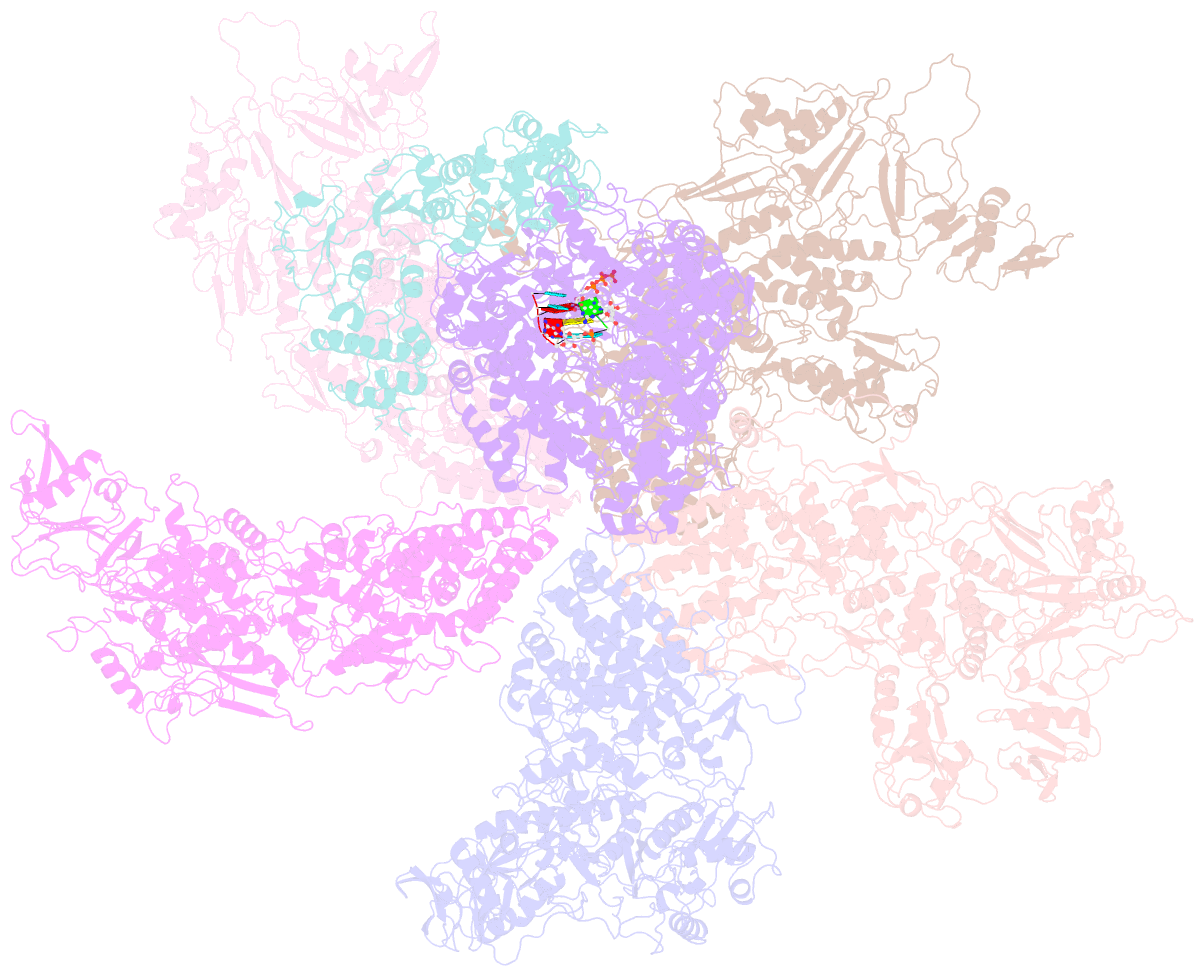
- Rdrp complex
- Reference
- Li X, Wang L, Wang X, Chen W, Yang T, Song J, Liu H, Cheng L (2020): "Structure of RdRps Within a Transcribing dsRNA Virus Provides Insights Into the Mechanisms of RNA Synthesis." J.Mol.Biol., 432, 358-366. doi: 10.1016/j.jmb.2019.09.015.
- Abstract
- RNA-dependent RNA polymerases (RdRps) catalyze RNA synthesis of RNA viruses. During initiation of RNA synthesis, the RdRp catalyzes the formation of the first dinucleotide, acting as primer for subsequent processive RNA elongation. Here, we present the structure of the RdRp complexes in the dinucleotide primed state in situ within a transcribing cypovirus under near physiological conditions using cryo-electron microscopy. The 3' end of RNA templates, paired RNA dinucleotide primer, incoming nucleotide, and catalytic divalent cations in the RdRp were resolved at 3.8 Å resolution. The end of the RNA template and the dinucleotide is buttressed by the aromatic tyrosine in a loop from the RdRp bracelet domain. Our structure reveals the interactions between the nucleotide substrates and the conserved residues during the RdRp initiation, and the coordinated structural changes preceding the elongation stage. In addition, it provides the direct evidence for existence of the slow step of the dinucleotide primed state in the viral RdRp transcription.